Articles
- Page Path
- HOME > J Mov Disord > Volume 12(1); 2019 > Article
-
Case Report
Treatment of Hemichoreoathetosis with Arrhythmic Proximal Tremor after Stroke: The Role of Zona Incerta as a Target for Deep Brain Stimulation -
Andrei Koerbel1
 , Augusto Radünz do Amaral2, Helena Bedatti Zeh2, Eduardo Wollmann2, Renata Fabiola Heil Koerbel3, Carla Moro4, Alexandre Luiz Longo4
, Augusto Radünz do Amaral2, Helena Bedatti Zeh2, Eduardo Wollmann2, Renata Fabiola Heil Koerbel3, Carla Moro4, Alexandre Luiz Longo4 -
Journal of Movement Disorders 2019;12(1):47-51.
DOI: https://doi.org/10.14802/jmd.18032
Published online: January 30, 2019
1Department of Neurosurgery, University of Joinville Region, and Neurological and Neurosurgical Clinic of Joinville, Joinville, Brazil
2Department of Neurosurgery, University of Joinville Region, Joinville, Brazil
3Intraoperative Monitoring, Neurological and Neurosurgical Clinic of Joinville, Joinville, Brazil
4Department of Neurology, Neurological and Neurosurgical Clinic of Joinville, Joinville, Brazil
- Corresponding author: Andrei Koerbel, MD, PhD, https://orcid.org/0000-0003-2658-8087 Department of Neurosurgery, University of Joinville Region, and Neurological and Neurosurgical Clinic of Joinville, Rua Placido Olimpio de Oliveira, 1244, Bucarein, Joinville, Santa Catarina 89202-165, Brazil / Tel: +55 47 34512525 / Fax: +55 47 34225935 / E-mail: andrei@apmelo.com.br
Copyright © 2019 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Deep brain stimulation (DBS) of the zona incerta has shown promising results in the reduction of medically refractory movement disorders. However, evidence supporting its efficacy in movement disorders secondary to hemorrhagic stroke or hemichoreoathetosis is limited. We describe a 48-year-old man who developed progressive hemichoreoathetosis with an arrhythmic, proximal tremor in his right arm following a thalamic hemorrhagic stroke. Pharmacological treatment was carried out with no change in the Abnormal Involuntary Movement Scale (AIMS) score after 4 weeks (14). After six sessions of botulinum toxin treatment, a subtle improvement in the AIMS score (13) was registered, but no clinical improvement was noted. The arrhythmic proximal movements were significantly improved after DBS of the zona incerta with a major decrease in the patient’s AIMS score (8). The response to DBS occurring after the failure of pharmacological and botulinum toxin treatments suggests that zona incerta DBS may be an alternative for postthalamic hemorrhage movement disorders.
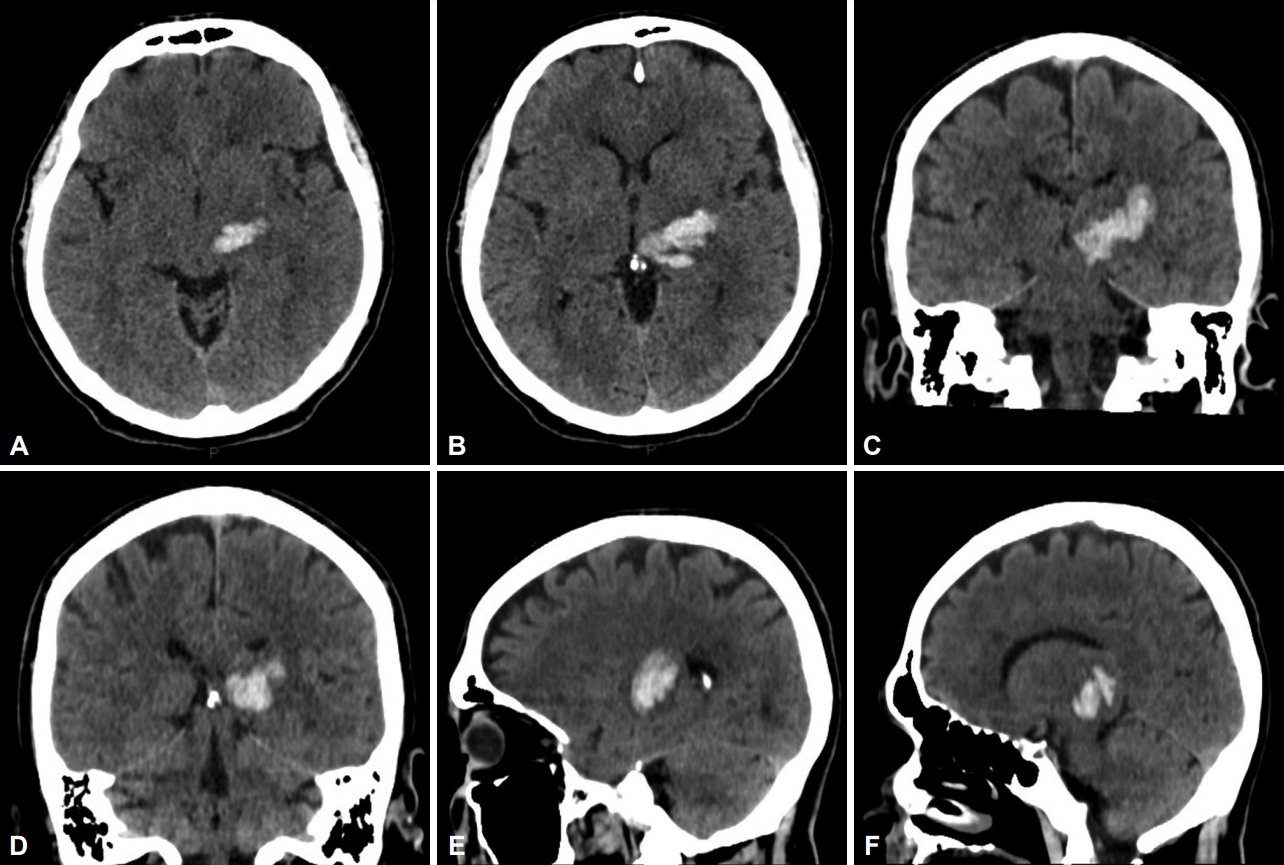
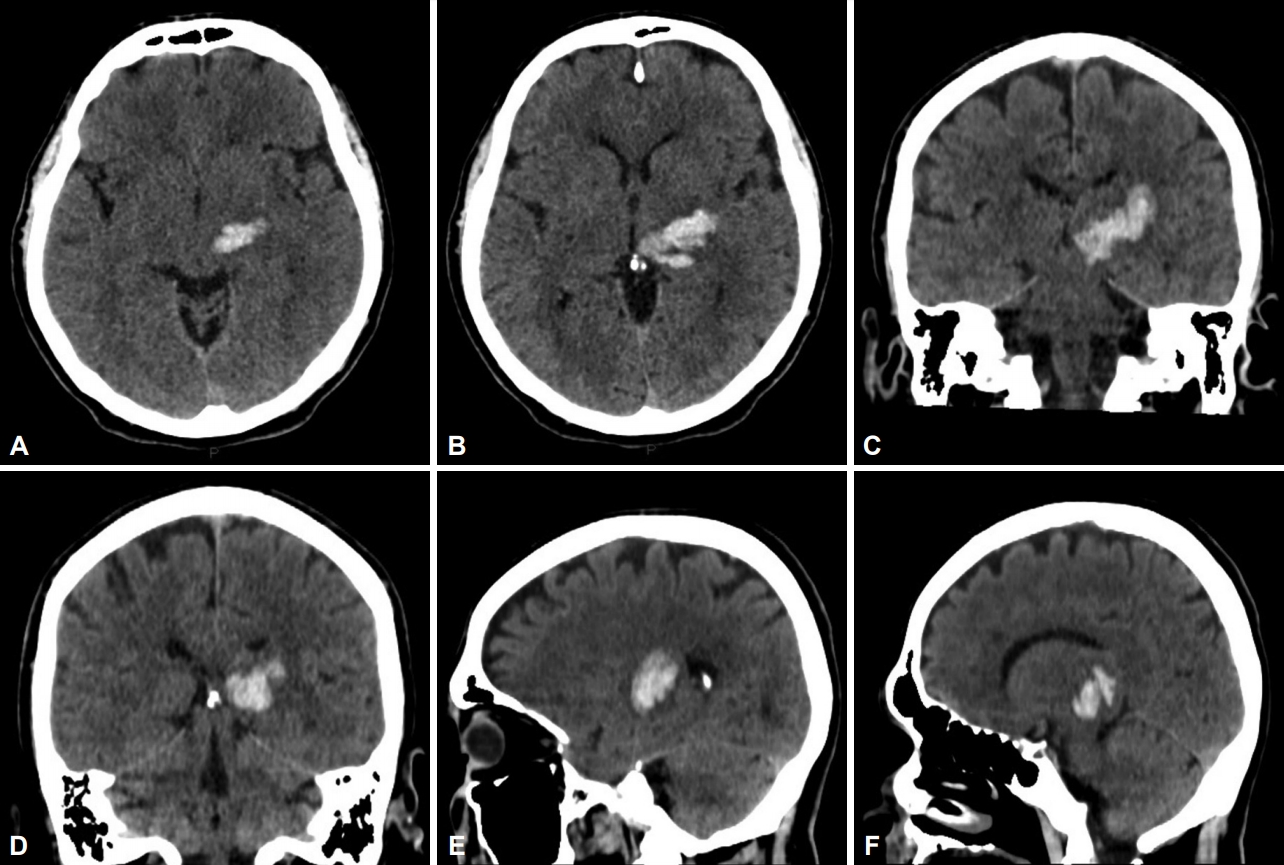
- A 48-year-old man with long-term hypertension and dyslipidemia presented with right hemiparesis and sensory loss. Clinical examination revealed moderate right hemiparesis with hemihypesthesia and mild dysarthria. A CT scan at the time showed a left thalamic hematoma involving the posterior thalamic region with compression and dislocation of the third ventricle to the opposite side (Figure 1).
- Cardiovascular evaluation revealed moderate left atrium enlargement and moderate diastolic dysfunction. Laboratory tests performed at the time were within normal limits.
- Forty-five days after hospital admission, the patient’s motor and sensory deficits improved significantly. He continued to display subtle dysarthria but began to present progressive involuntary choreoathetoid movement in his right arm. The movement was arrhythmic and hyperkinetic, with a low frequency (2–3 Hz) and large amplitude. This continuous movement occurred in distal and proximal regions; the movement improved but did not disappear with the rest. In addition, it worsened with actions of the arm, and it presented associated dystonic and myoclonic components as well as moments that resembled ballism. The patient’s right hand showed more choreic finger movements; however, this could have been pseudoathetosis because the patient still had sensory deficits. The patient had no control of these abnormal movements but noticed exacerbation by anxiety. The patient’s Abnormal Involuntary Movement Scale (AIMS) at that time was 14.
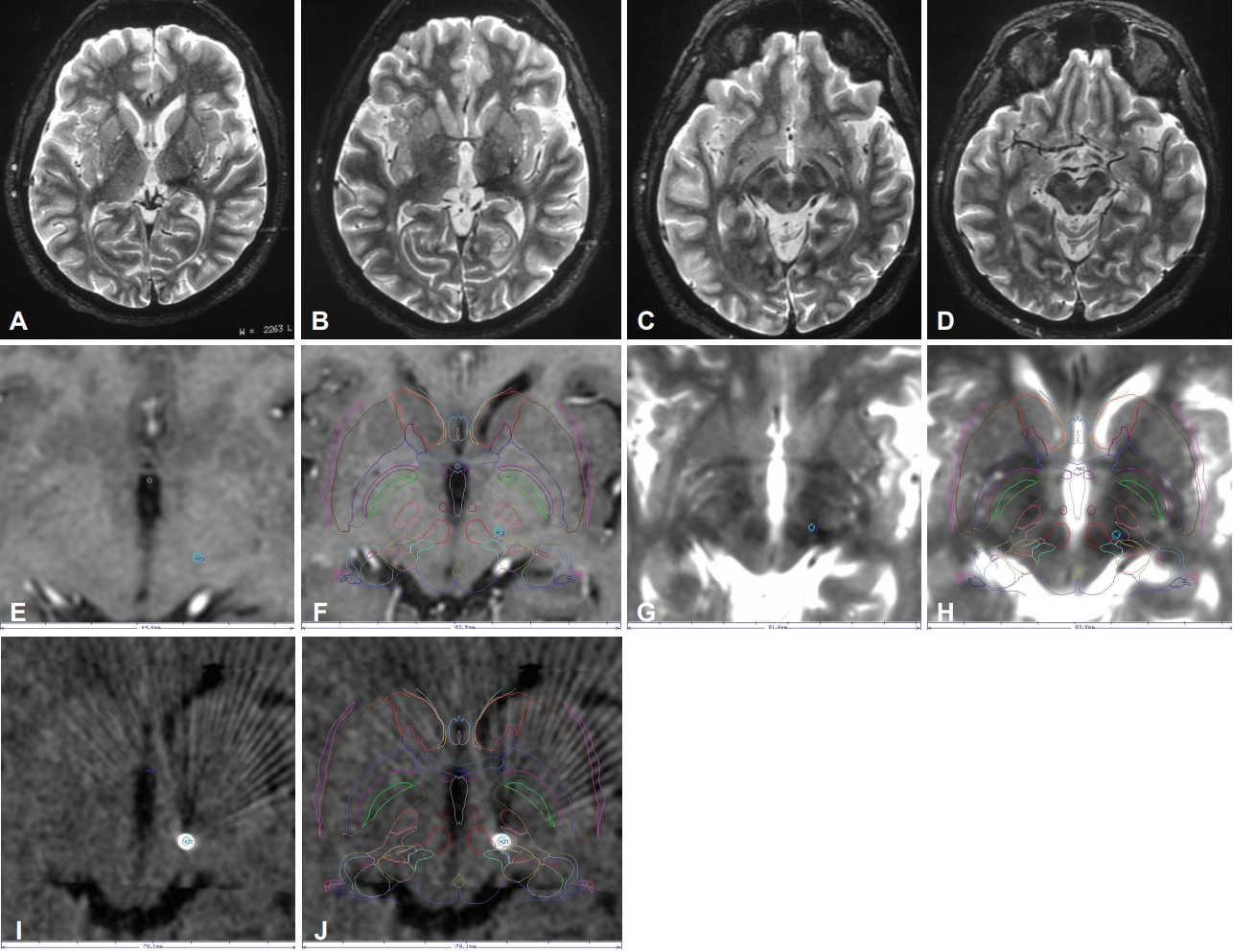
- After a week, a new re-evaluation showed persistent movement disorder. Risperidone and haloperidol were administered, but there was no improvement in the AIMS score (14). Due to inadequate clinical response and severe functional impairment, treatment with botulinum toxin was offered. The patient underwent 6 sessions of botulinum toxin therapy and was evaluated after each session to determine the level of improvement in the movement disorder. At the end of the treatment, there was only a subtle improvement in AIMS score (14 to 13). Therefore, no significant clinical improvement was noticed, and the patient’s quality of life did not improve. No adverse effects were noted by the patient in any session. MRI performed 2 years after the stroke showed gliosis and attenuation of white matter extending from the left thalamus to a region between the subthalamic and the red nuclei (Figure 2A-D).
- DBS of the zona incerta was then offered as an alternative for management of the movement disorder. Microelectrode recording was performed intraoperatively to redefine the structures surrounding the subthalamus. Typical activity of the left thalamus, zona incerta, subthalamic nucleus and substantia nigra was recorded. A St Jude DBS lead (Abbott St. Jude Medical DBS System, Austin, TX, USA) was implanted with the first electrode in the transition between the subthalamic nucleus and the zona incerta, and electrodes 2 and 3 were located in the zona incerta itself. This procedure was carried out unilaterally. A nonrechargeable pulse generator was implanted.
- The patient then underwent a stereotactic CT on the day after frame removal for evaluation of electrode positioning (Figure 2E-J).
- The device was turned on fifteen days after the surgery and tests were performed. At the first moment, a high frequency (190 Hz) and double monopolar stimulation appeared to offer some response, while a lower frequency and single monopolar stimulation showed no improvement. A slight improvement was further noticed in the weeks following the start of the stimulation. In the follow up, the frequency was reduced to maintain a working battery. Nonetheless, the symptoms continued to improve despite the frequency reduction.
- After the optimal stimulation parameters were reached, no side effects were observed. There was gradual suppression of movement. Remarkable improvement of the movement disorder was noticed from the sixty-sixth month onwards. Further improvement occurred in the following months. The patient did not present any side effects such as gait, speech disorder or weakness.
- One year after the procedure, the symptoms abruptly recurred. The device evaluation indicated that the pulse generator battery was depleted. The patient was submitted to a new procedure to implant a rechargeable St Jude generator (Abbott St. Jude Medical DBS System). The generator was programmed the same way as before. The hemichoreoathetosis with arrhythmic proximal tremor slowly decreased in the following months, and optimal results were achieved six months after the generator replacement.
- Evaluation performed at 1, 6, 12 and 24 months after the first surgery revealed a major improvement in the AIMS score (13 to 8) with a significant reduction in the patient’s symptoms as noted at physical examination (Supplementary Video 1 in the online-only Data Supplement). The postoperative video was taken at 24 months after the surgery. The patient also reported an important change in his quality of life due to an improvement in his basic personal care (e.g., bathing, dressing and feeding). Three years after the first surgery there was no recurrence of symptoms.
- This study obtained full informed consent from the patient for publication.
CASE REPORT
- Thalamic lesions, as in this case, have been previously associated with involuntary movement disorders, such as choreoathetosis and hemidystonia, which have been described in other studies [2,4]. According to Lee et al. [5], the occurrence of both motor incoordination and tremor relies on the disturbance of data (peripheral sensory pathways, basal ganglia and cerebellum) transmitted from the thalamus to the cerebral cortex.
- To our knowledge, this is the first study to describe DBS of the zona incerta in the treatment of hemichoreoathetosis with arrhythmic proximal tremor. The distinctive element of our case is the control of dyskinetic movements despite the failure of pharmacological and botulinum toxin treatments.
- The target of choice for DBS in this case was particularly challenging due to the complexity of the patient’s movement disorder and the lack of evidence on DBS treatment for hyperkinetic movement following intracerebral hemorrhage. However, recent studies have presented conflicting evidence regarding its use for movement disorders secondary to stroke. In two similar series [6,7] in which pallidal stimulation was performed after cerebral infarction, vastly conflicting outcomes were noted. Witt et al. [6] reported a limited benefit in all cases (nonsignificant increase or unsustainable improvement in Burke-Fahn-Marsden Dystonia Rating Scale motor score); Fuller et al. [7] noticed a major improvement in the patient’s quality of life despite the large striatal infarction, with clear improvements in pain, amelioration of dystonic tremor, absence of trunk spasm, writing rehabilitation and normal speech.
- The left thalamus itself would not have been a suitable target due to the formation of a glial scar in the area previously affected by the hematoma. It has been postulated that an area of glial scar tends to be electrically inactive; therefore, it is not a viable option for DBS [8]. Additionally, according to Fytagoridis et al. [9], the disposition of white matter tracts in the posterior subthalamic area would make the zona incerta a more potent area for stimulation.
- The choice of targeting the zona incerta was based on physiological knowledge, as the caudal portion of zona incerta is responsible for the generation of axial and proximal limb movements [10]. According to Fytagoridis et al. [9], the caudal portion of the zona incerta transmits GABAergic input from the basal ganglia to the cerebello-thalamo-cortical circuits. Therefore, a targeted DBS in this area could downregulate or inhibit abnormal thalamic oscillations.
- The data collected in this report suggests that chronic stimulation of zona incerta can provide long-term improvements to choreoathetoid movement following thalamic hemorrhage with no significant side effects. There is a latency period of some months until the zona incerta stimulation becomes effective in controlling hemichoreoathetosis with arrhythmic proximal tremor.
- Further case studies and prospective multicenter studies in controlled scenarios are necessary to extend and validate the role of zona incerta as the target of DBS in similar scenarios.
DISCUSSION
Supplementary Materials
Supplementary Video Legends
- We thank Mr. Daniel Cosme for his indispensable assistance in all DBS surgeries, and for preparing and providing the pictures of Figure 2 in this manuscript.
Acknowledgments

- 1. Tokgoz S, Demirkaya S, Bek S, Kasıkcı T, Odabasi Z, Genc G, et al. Clinical properties of regional thalamic hemorrhages. J Stroke Cerebrovasc Dis 2012;22:1006–1012.ArticlePubMed
- 2. Calabrò RS, Polimeni G, Gervasi G, Bramanti P. Postthalamic stroke dystonic choreoathetosis responsive to tetrabenazine. Ann Pharmacother 2011;45:e65.ArticlePubMed
- 3. Álvarez M, Quintanal N, Díaz A, Prince J, García I, Carballo M, et al. Dystonia and tremor secondary to thalamic infarction successfully treated with thalamotomy of the ventralis intermedius nucleus. Mov Disord 2014;29:1188–1190.ArticlePubMed
- 4. Weise D, Hammer N, Rumpf JJ, Fritzsch D, Meixensberger J, Schwarz J, et al. Unilateral multi-target deep brain stimulation in hemidystonia and hemichoreoathetosis following ischemic thalamic stroke. Basal Ganglia 2016;6:153–156.Article
- 5. Lee MS, Marsden CD. Movement disorders following lesions of the thalamus or subthalamic region. Mov Disord 1994;9:493–507.ArticlePubMed
- 6. Witt J, Starr PA, Ostrem JL. Use of pallidal deep brain stimulation in postinfarct hemidystonia. Stereotact Funct Neurosurg 2013;91:243–247.ArticlePubMed
- 7. Fuller J, Prescott IA, Moro E, Toda H, Lozano A, Hutchison WD. Pallidal deep brain stimulation for a case of hemidystonia secondary to a striatal stroke. Stereotact Funct Neurosurg 2013;91:190–197.ArticlePubMed
- 8. Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull 1999;49:377–391.ArticlePubMed
- 9. Fytagoridis A, Sandvik U, Astrm M, Bergenheim T, Blomstedt P. Long term follow-up of deep brain stimulation of the caudal zona incerta for essential tremor. J Neurol Neurosurg Psychiatry 2012;83:258–262.ArticlePubMed
- 10. Plaha P, Ben-Shlomo Y, Patel NK, Gill SS. Stimulation of the caudal zona incerta is superior to stimulation of the subthalamic nucleus in improving contralateral parkinsonism. Brain 2006;129(Pt 7):1732–1747.ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations

- Deep brain stimulation for movement disorders after stroke: a systematic review of the literature
Mitch R. Paro, Michal Dyrda, Srinath Ramanan, Grant Wadman, Stacey-Ann Burke, Isabella Cipollone, Cory Bosworth, Sarah Zurek, Patrick B. Senatus
Journal of Neurosurgery.2022; : 1. CrossRef - Deep brain stimulation for post-thalamic stroke complex movement disorders
A. Macerollo, B. Hammersley, M. Bonello, J. Somerset, D. Bhargava, K. Das, J. Osman-Farah, P. R. Eldridge, S. H. Alusi
Neurological Sciences.2021; 42(1): 337. CrossRef - Neurologic Manifestations of Systemic Disease: Movement Disorders
Giulietta M. Riboldi, Steven J. Frucht
Current Treatment Options in Neurology.2021;[Epub] CrossRef
Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite