Update on Current Technologies for Deep Brain Stimulation in Parkinson’s Disease
Article information
Abstract
Deep brain stimulation (DBS) is becoming increasingly central in the treatment of patients with Parkinson’s disease and other movement disorders. Recent developments in DBS lead and implantable pulse generator design provide increased flexibility for programming, potentially improving the therapeutic benefit of stimulation. Directional DBS leads may increase the therapeutic window of stimulation by providing a means of avoiding current spread to structures that might give rise to stimulation-related side effects. Similarly, control of current to individual contacts on a DBS lead allows for shaping of the electric field produced between multiple active contacts. The following review aims to describe the recent developments in DBS system technology and the features of each commercially available DBS system. The advantages of each system are reviewed, and general considerations for choosing the most appropriate system are discussed.
The development of deep brain stimulation (DBS) over 30 years ago has provided a means of relieving the conditions of patients with a variety of movement disorders and has since become increasingly important in the treatment of Parkinson’s disease (PD) [1,2]. The two most common targets for DBS for PD are the globus pallidus internus (GPi) and the subthalamic nucleus (STN). While both targets are effective for reducing levodopa-induced dyskinesias and improving mobility, STN-DBS has the added benefit of reducing the daily levodopa requirement; on the other hand, the GPi is considered a safer target from a psychiatric and cognitive perspective [3-5].
DBS surgery has been shown to be effective for improving the motor symptoms and quality of life of PD patients; however, there are some symptoms that may be resistant or refractory to stimulation. For example, gait, speech and postural dysfunction tend to deteriorate independently and at a faster rate than appendicular symptoms [6]. STN-DBS in particular is known to exacerbate stuttering and hypophonia in PD patients [7]. Additionally, stimulation at contacts residing within specific regions of the STN may have differential effects on axial and appendicular symptoms. For example, stimulation of the dorsal half of the lateral STN been shown to improve step velocity, length and balance compared to stimulation of ventral regions [8]. The potential of STN-DBS to adversely affect axial symptoms is exemplified by a case report in which de novo freezing of gait (FOG) was produced with stimulation of the anteromedial region of the STN [9]. By contrast, GPi DBS is associated with fewer axial adverse events although this observation mainly comes from uncontrolled open studies [6]. These findings demonstrate potential challenges that may arise when optimizing stimulation settings for individual patients.
The therapeutic potential of DBS relies heavily on ideal placement of the DBS electrode within the target nucleus. While multiple localization techniques, such as image guidance, microelectrode recordings, and clinical evaluation, may be implemented during the surgical procedure, small deviations from the planned trajectory can result in a reduced therapeutic window for stimulation. The STN is a small, lens-shaped nucleus measuring approximately 8 mm in length and 6 mm in width that is divided into dorsolateral sensorimotor, associative and medioventral limbic regions [10]. Studies have shown that optimal implantation of the electrode into the dorsolateral region of the STN is necessary to produce improvements in motor symptoms while avoiding stimulation-related side effects [11,12]. DBS lead revisions are uncommon due to the risk of surgical complications; thus, clinicians may have to rely on programming strategies to optimize therapeutic benefit in the case of suboptimally placed DBS electrodes.
Obtaining an optimal clinical response while minimizing stimulation-related side effects can be achieved in the majority of cases by manipulating stimulation parameters, such as electrode polarity, frequency, pulse width, and voltage as well as by shaping the volume of tissue activation (VTA). For example, bipolar stimulation can narrow the shape of the VTA to avoid the fibers of the internal capsule or other eloquent structures. Furthermore, some studies have reported improvements in gait with switching to low-frequency (60 Hz) high-voltage stimulation [13,14] as opposed to standard protocols implementing high frequency stimulation (HFS). Recently, technological advances in DBS systems have given rise to additional programming strategies that allow for further flexibility in manipulating stimulation parameters. Such advancements may aid clinicians in optimizing program settings for patients with complex Parkinsonian symptoms or suboptimally positioned DBS leads. The present review will discuss new techniques for stimulation implemented by the latest generation of DBS systems with a focus on the unique features of each system.
UPDATE ON STIMULATION
The goals of effective DBS programming in order of importance are to maximize clinical benefit, avoid stimulation-related side effects, and minimize current consumption [15]. In general, stimulation through an optimally positioned DBS electrode should produce a significant clinical benefit with relatively low energy consumption. Monopolar stimulation, in which one (or two in case of ‘double monopolar’) of the electrode contacts serves as a cathode against the implantable pulse generator (IPG) case, produces a radial diffusion of current around the active contact. The radius of the VTA increases with increasing amplitude (voltage or current). Conversely, in bipolar stimulation, one contact serves as the cathode while another serves as the anode. This produces a narrower VTA with the greatest field strength centered around the cathode. Bipolar stimulation may be helpful in cases where the VTA produced by monopolar stimulation violates surrounding eloquent structures leading to side effects. Interestingly, bipolar stimulation has been found to be associated with longer battery life than double monopolar and even monopolar stimulation using a voltage-constant IPG [16]. The therapeutic benefit from STN-DBS in PD patients has been observed with stimulation frequencies greater than 50 Hz, and clinical improvements in rigidity and bradykinesia are maximized at approximately 130 Hz [17]. Conversely, stimulation at 5 Hz has been observed to worsen symptoms of rigidity and bradykinesia [17].
Constant current
Until recently, all DBS IPGs utilized single-source, constant voltage-driven stimulation. The consequence of this design is that the impedance of the DBS electrode at the contact-tissue interface determines the magnitude of current flow to the neural elements, and this impedance may change over time (e.g., after IPG replacements). Current Medtronic IPGs can provide either voltage-constant or current-constant stimulation, but the former is usually preferred as more programming options are available. Currently, the majority of manufacturers only produce current-constant IPGs.
Pulse width
Pulse width is usually kept at the lowest duration of 60 μs unless there is lack of clinical benefit with increasing amplitude [15]. While the amount of current required to excite neural tissue decreases with increased pulse width [18], the amplitude can be more easily manipulated with most IPGs [15]. Shortening the pulse width < 60 μs has shown some utility in increasing the therapeutic window of stimulation, namely, the range of amplitudes that produce clinical benefit without side effects [19]. Pulse widths < 60 μs are only possible with use of newer IPGs, some of which are compatible with electrodes produced by different manufacturers [20]. Shortening the pulse width below 60 μs was shown to reduce stimulation-related side effects and increase the therapeutic window of stimulation in two patients with essential tremor (ET) when they were switched from a Medtronic IPG to a Boston Scientific Vercise IPG [20].
Frequency
Varying stimulation frequency has recently been explored for treating gait and axial symptoms in PD that responded poorly to the traditional HFS of 130 Hz. DBS targeting the pedunclopontine nucleus is an experimental procedure previously investigated to address FOG in PD patients, and its optimal stimulation settings utilized lower frequencies than other targets, most commonly frequencies < 60 Hz [21]. Low-frequency stimulation (LFS) was hypothesized to provide better control of axial symptoms, including FOG, even when delivered to the STN [13]. In a small study of 13 PD patients with FOG and prominent axial symptoms, LFS was found to improve gait and reduce freezing episodes while maintaining a reduction in the Unified PD Rating Scale (UPDRS) motor score although the majority of patients had to increase their levodopa dose to mitigate worsening Parkinsonian symptoms [13]. Similarly, LFS was found to improve gait and axial symptoms in a cohort of 14 patients, and the optimal contacts for LFS were located ventral to the contacts for optimal HFS of 130 Hz [22]. Variable frequency stimulation (VFS) involves alternating HFS with LFS. Jia et al. [23] proposed that VFS may serve as an effective strategy for treating FOG and axial symptoms in PD patients while maintaining optimal treatment of appendicular symptoms. This was exemplified in a case of one PD patient with FOG who improved with VFS. Subsequently, a small case study of 4 PD patients with FOG symptoms demonstrated that VFS improved gait speed and reduced episodes of FOG [24]. Additionally, an acute open-label study found that high stimulation frequency of 10 kHz was reported to yield good mobility with fewer stimulation-induced paresthesias and speech disturbances than the traditional stimulation frequency of 130 Hz [25].
Temporal fractionation
Temporal fractionation involves using two separate programs in an alternating fashion through a single DBS electrode. The two programs may have different amplitudes, polarities, and pulse widths; however, the frequency must be the same, with a maximum allowed frequency of 125 Hz (i.e., the maximum IPG output is 250 Hz). This method of stimulation allows for different areas of the target nucleus to be stimulated simultaneously. This technology is generally known as ‘interleaving stimulation’, as introduced by Medtronic; a similar technology is called ‘multi-stim set’ in Abbott devices (see below).
Case reports have described interleaving stimulation to be effective for treating refractory dystonia [26] and for treating tremor predominant symptoms in a patient with comorbid PD and ET [27]. This technology may be particularly helpful in cases in which a DBS electrode is suboptimally placed. Miocinovic et al. [28] described 3 patients, each of whom had one DBS lead that was suboptimally positioned within the STN. Stimulation using various monopolar and double monopolar programs improved some symptoms but worsened others and caused stimulation-related side effects. Use of interleaving programs was effective for providing stimulation of the desired areas while avoiding surrounding structures. Similarly, Zhang et al. [29] reported a series of 12 patients in whom multiple trials of conventional stimulation were ineffective in controlling various symptoms. Prior to implementing interleaving stimulation, the patients’ complaints included dysarthria, stimulation-related dyskinesias, gait disturbances, and incomplete control of Parkinsonian symptoms. MRI evaluation revealed suboptimal placement of the affected lead in most patients in this series; however, adequate control of symptoms was able to be achieved in all with interleaving stimulation. In addition to avoiding stimulation-related side effects, interleaving stimulation has been shown to improve dyskinesias in a subset of PD patients. Interestingly, this effect was achieved with activation of a dorsal contact residing in the zona incerta, just outside the STN [30]. These reports exemplify how interleaving provides greater flexibility in programming, enabling the clinician to better balance Parkinsonian symptoms and stimulation-related side effects.
Current fractionation
The concept of current fractionation involves multiple independent current sources that apply constant current through each electrode, enabling the clinician to directly control the amount of current delivered to the surrounding tissues. Control of current flow through each contact individually is termed multiple independent current control (MICC) in Boston Scientific IPGs. Utilizing this paradigm, current can be distributed in a controlled fashion between multiple contacts, allowing for the VTA to be adjusted to a desired shape to fit the target region [31]. Current fractionation is different than temporal fractionation, which uses technologies such as interleaving (see above).
MICC is not possible on Abbott devices, which use a different—less versatile—method called coactivation. Coactivation allows for multiple contacts to be stimulated as if they were a single electrode (see below). In a modeling study, coactivation was shown to be associated with lower power consumption than MICC due to lowered impedance at the electrode-tissue interface [32].
Computational models have demonstrated that direct current steering may have the potential to stimulate desired neuronal populations even in the case of suboptimally placed DBS electrodes [31,33]. In fact, based on these models, selective therapeutic stimulation may be achieved across a range of electrode locations [33]. The utility of this method of stimulation has been demonstrated in some case series and case reports. For example, current steering to shift the stimulation toward a more proximal contact, changing the shape of the VTA to a tear-drop-shaped distribution has been used in a patient who developed dyskinesias with increasing amplitude at his most used therapeutic contact. This effectively alleviated the patient’s Parkinsonian symptoms without eliciting dyskinesias [34].
However, targeting with such accuracy would require detailed information on the location of the electrode within the target nucleus of the individual and consequently may complicate the programming process. The authors suggest that visualization software could help guide clinicians by means of patient-specific computational models during the programming process [33]. For example, the GUIDE system by Boston Scientific (Valencia, CA, USA) is a three-dimensional visual software that provides information about the location of the DBS lead based on preoperative MRI and postoperative CT images. The VTA can be modeled when the stimulation settings are selected, allowing clinicians to visualize the resulting VTA in relation to surrounding structures. Use of this programming software was shown to shorten the duration of the initial programming session by a mean of 75% [35].
Anodic stimulation
Traditionally, cathodic stimulation has been favored, since activation of myelinated fibers requires 3–8 times more stimulation strength when the active contact acts as an anode [36]. For this reason, first-generation DBS systems were designed such that the IPG case served as the anode while the active contact served as the cathode. Theoretically, the active contact on a DBS electrode can act as either a cathode or an anode. Cathodic and anodic stimulation are believed to differentially affect neural tissue depending on the orientation of the fibers, such that passing fibers are activated by cathodal stimulation and orthogonally oriented fibers are activated by anodic stimulation [37]. With the recent development of second-generation DBS systems that allow for increased flexibility in stimulation parameters, the effects of anodic stimulation in comparison to cathodal stimulation have been studied [38,39]. Kirsch et al. [38] studied anodic stimulation in 10 PD patients and reported higher side effect thresholds and lower UPDRS III motor scores with anodic stimulation than with cathodic stimulation. Another study involving 10 PD patients found an increased therapeutic window and higher side effect threshold with anodic stimulation [39]. However, anodic stimulation was not able to control tremors in two patients, and anodic stimulation is also known to result in higher battery consumption [39].
Semibipolar stimulation
In semibipolar stimulation, the anode is divided equally between the IPG case and another contact on the DBS electrode. Based on clinical experience, this configuration is believed to be useful for avoiding stimulation-related side effects. Soh et al. [39] compared the effects of semibipolar stimulation to bipolar, monopolar cathodal and monopolar anodic stimulation in a study group of 10 PD patients. Semibipolar stimulation was found to have a significantly higher side effect threshold than cathodal stimulation; furthermore, semibipolar stimulation was found to have a lower battery consumption than bipolar stimulation [39].
Directional leads
DBS lead design has exhibited significant growth in recent years with the development of commercially available directional leads. The traditional DBS lead structure consists of 4 contacts of 1.5 mm in length separated from one another by interspaces of 1.5 mm in the case of the Medtronic 3387 model and 0.5 mm in the case of the Medtronic 3389 model lead. As described previously, monopolar stimulation produces a spherical electric field that diffuses radially from the active contact. Directional leads, on the other hand, contain multiple contacts in a radial distribution around the shaft of the DBS lead. Each contact may be stimulated individually or in combination by means of temporal fractionation (Abbott devices) or current fractionization (MICC, Boston Scientific). Passing current through individual contacts enables current steering along a vector perpendicular to the lead. Commercially available directional leads have a circumferential ring configuration made up of 3 individual contacts. Stimulation through all 3 contacts simultaneously produces an electric field equivalent to that of monopolar stimulation through a traditional DBS lead. Importantly, the smaller size of each individual contact leads to a greater electric field density at the contact-tissue interface; therefore, less current is required to produce a therapeutic effect. This feature may potentially contribute to preserved battery life.
The advantage of the directional lead design was first proposed by computational models [40,41]. One of these models suggested that current steering through segmented leads could shift the center of the VTA as much as 1.0–1.3 mm [42]. While this distance may seem insufficient to compensate for a poorly positioned DBS lead, studies in human subjects have demonstrated that directional stimulation through a most favorable contact could increase the therapeutic window for stimulation and increase the current threshold for producing side effects [43-45].
Two initial studies performed in PD patients were intraoperative alone [44,46]. Pollo et al. [44] calculated that the average therapeutic window was 41.3% wider than that of omnidirectional stimulation, and the average therapeutic current was 43% less for the best directional stimulation than for omnidirectional stimulation. Contarino et al. [46] found similar results in a double-blind intraoperative study in eight PD patients by means of a non-commercialized 32-contact electrode. Dembek et al. [43] investigated the clinical benefit of directional stimulation in a chronic double-blind crossover study in which patients received a trial of best directional stimulation compared to omnidirectional stimulation. This group found no difference in motor outcomes between the two conditions other than a significant positive difference in hand rotation in the directional condition.
The utility of directional leads for STN-DBS was demonstrated in the PROGRESS study in which 234 PD patients were implanted with Abbott directional leads targeting the STN [47]. For the first three months, patients received traditional omnidirectional stimulation, and during the following 3-month period, patients were switched to directional stimulation. The results of this prospective, single-arm crossover study yielded a significant mean 41% increase in the therapeutic window for stimulation with directional stimulation. Additionally, the amount of current required to produce therapeutic effects was reduced by 39% [47]. The capability and limitation of directional leads to steer current in the case of a misplaced DBS lead is depicted in Figure 1.

A: Stimulation of the dorsolateral STN through an ideally positioned DBS lead. B: Current steering through a directional DBS lead is unable to compensate for a misplaced lead that resides outside the target nucleus. C: Use of current steering through a directional lead that is positioned slightly posterior to the STN is capable of steering current toward the dorsolateral STN. STN: subthalamic nucleus, DBS: deep brain stimulation.
One potential disadvantage of directional leads is the increased complexity of programming due to the addition of multiple contacts directed in different orientations. Fortunately, monopolar review can be performed in a similar fashion as with traditional lead types, and directional stimulation may be implemented only if needed to address side effects or complex symptoms. Algorithms implementing anatomical models based on MR and diffusion tensor imaging to automate programming of directional lead have been proposed [48]. However, access to such technology is likely to be a limiting factor for many community neurologists.
UPDATE ON DEVICES
DBS system manufacturers
Medtronic
Medtronic moved its headquarters from Minneapolis, MN, USA to Dublin, Ireland, after acquiring Covidien, a rival medical device company, in 2015. The Medtronic 3382 DBS lead and the ITREL IPG was the first DBS system to gain U.S. Food and Drug Administration (FDA) approval in the United States for treatment of ET and tremor-dominant PD in 1997. In 2002, this system became FDA-approved for treatment of bradykinesia and rigidity in PD. The early IPGs were single channel devices, such that bilateral DBS required implantation of 2 IPGs (e.g., Soletra was approved in South Korea in 2001). The Kinetra IPG by Medtronic, which became available in 1998, was the first dual-channel IPG (never launched in South Korea). In 2008, the Kinetra IPG was replaced by the Activa Primary Cell (PC), which was approved in South Korea in 2010. An IPG serving a single channel (Activa SC) was also subsequently produced and approved in many countries, including in South Korea in 2011. In 2015, the FDA approved Medtronic DBS systems for full-body MRI when certain safety conditions were met. In addition to the commercially available Activa IPGs, Medtronic launched a novel system named Activa PC+S for research use in 2013. This system enables the sensing and recording of select brain activity while simultaneously providing targeted DBS therapy. Following the success of this sensing-enabled system, a commercially available form named Percept PC was released in January 2020. This new IPG is using the BrainSense technology that enables physicians to track patient-specific brain signals and correlate these with patient-recorded symptoms and treatment side effects. Additionally, the Percept PC system allows for 3T full-body MRI scan has a lower pulse width range lower limit and an improved battery longevity than the Activa PC [49]).
Abbott
St. Jude Medical (St. Paul, MN, USA) was the first medical device company to develop constant-current DBS systems, the Libra and Libra XP. The Libra DBS system was tested for efficacy in PD, with 136 patients implanted between October 2005 and April 2009 in a multicenter trial involving 15 centers [50]. Additionally, the Libra system was used to treat ETs in 127 patients at 12 investigational sites [51]. These trials were designed to establish the safety and efficacy of this DBS system for the purposes of gaining FDA-approval and did not compare constant-current to constant-voltage devices. The Libra system was FDA-approved as an investigational device in 2012 to investigate the efficacy of subcallosal cingulate gyrus stimulation for the treatment of refractory depression in a multicenter trial [52]. Subsequently, the rechargeable Brio DBS system was granted FDA approval to treat PD and ETs in 2015.
Despite gaining FDA approval, the Libra, Libra XP, and Brio systems were never commercialized and are not available for clinical use in the United States. They are instead used in many other countries (e.g., in South Korea, Libra was launched in 2013 and Brio in 2016), St. Jude Medical was later acquired by Abbott Laboratories (Abbott Park, IL, USA). The Infinity DBS system was the first St. Jude/Abbott DBS system to be commercially available in the United States. The Infinity system was also the first FDA-approved system to utilize a directional lead, which was granted in 2016 (same year in South Korea). This system can be programmed using an iPad Mini (Apple, Cupertino, CA, USA), and patients can connect the implant with a paired iPod Touch (Apple). Communication between the IPG device and these programmers occurs via Bluetooth, and no physical contact is necessary. More recently, Abbott received FDA approval for a software upgrade allowing patients implanted with the Infinity DBS system to receive full-body MRI.
Boston Scientific
Boston Scientific launched the Vercise DBS system in Europe in 2012. This system was evaluated in the VANTAGE trial, a multicenter, open-label, nonrandomized study conducted at 6 centers in 6 European countries [53]. Forty patients received bilateral STN-DBS using an eight-contact DBS lead and a multisource, constant-current, rechargeable DBS IPG. Measures of clinical improvement and the incidence of stimulation-related side effects were similar to those in previous trials of DBS for PD [53]. The Vercise DBS system was granted FDA approval in December 2017 based on the results of the INTREPID study [54]. In the multicenter, double-blind, randomized, controlled trial, 160 subjects received bilateral STN-DBS with the Vercise system and then were randomized to receive 12 weeks of active or sham stimulation. The study achieved its primary endpoint of change in the number of hours spent with good mobility based on patient diaries, and the results at one year of follow up reported an improvement in the UPDRS III motor score of 49.2% compared to baseline [55]. A few years later in Europe in January of 2019, the Vercise Gevia IPG with the Cartesia directional DBS lead became FDA approved, and later that year, the FDA granted labeling of the ImageReady MRI Vercise Gevia DBS system, enabling patients implanted with the device to qualify for full-body MRI. In South Korea, the Vercise PC was approved in 2017, and Gevia was approved one year later.
PINS Medical
PINS Medical Co., Ltd. (Beijing, China) was established in 2008, and the PINS DBS system was developed as less-costly alternative to Medtronic devices. As of October 2016, 5,000 patients have been implanted with PINS DBS systems throughout China. The PINS IPG models resemble those of Medtronic, with single-channel, dual-channel, and dual-channel rechargeable options. The PINS DBS electrodes also resemble those of Medtronic with four 1.5 mm-long contacts spaced 1.5 mm or 0.5 mm apart. The PINS DBS system, utilizing a single-channel IPG, was evaluated in 40 patients in two centers in China in an open-label trial [56] in which 35 patients received bilateral and 5 patients received unilateral STN-DBS between November 2009 and December 2011. The PINS IPG was the first device to allow for VFS, in which stimulation at two different frequencies may be interleaved. VFS is proposed to be helpful in treating axial symptoms in PD patients. In a small case series of 4 PD patients with FOG, VFS was observed to improve the UPDRS III motor score by a mean of 14% more than HFS, and gait speed was increased by a mean of 45% in 3 of 4 patients [57].
SceneRay
SceneRay Co., Ltd. (Suzhou, China) was initiated in 2015, and in 2019, the SceneRay DBS system obtained registration with the Chinese Food and Drug Administration. The SceneRay model 1180 IPG is a dual-channel device that is capable of remote wireless programming. The currently available electrode models contain four contacts of 1.5 mm in length and are separated from one another by 0.5, 1.0, or 1.5 mm intervals. SceneRay has also designed an electrode to specifically to target the nucleus accumbens and anterior limb of the internal capsule [58]. The lead has four contacts of 3.0 mm in length; the two ventral-most contacts are separated by 2 mm, and the more dorsal contacts are separated by 4 mm. Both SceneRay and PINS promote web-based, remote, wireless DBS programming systems in which patients may have their DBS settings adjusted at home by a clinician remotely located in a hospital or clinic. While the advantages of remote programming capabilities include increased access to neurologists and reduced travel costs for patients, the programming sessions tend to be lengthier and susceptible to network instability and communication errors [59].
Commercially available DBS pulse generators
Medtronic Activa series
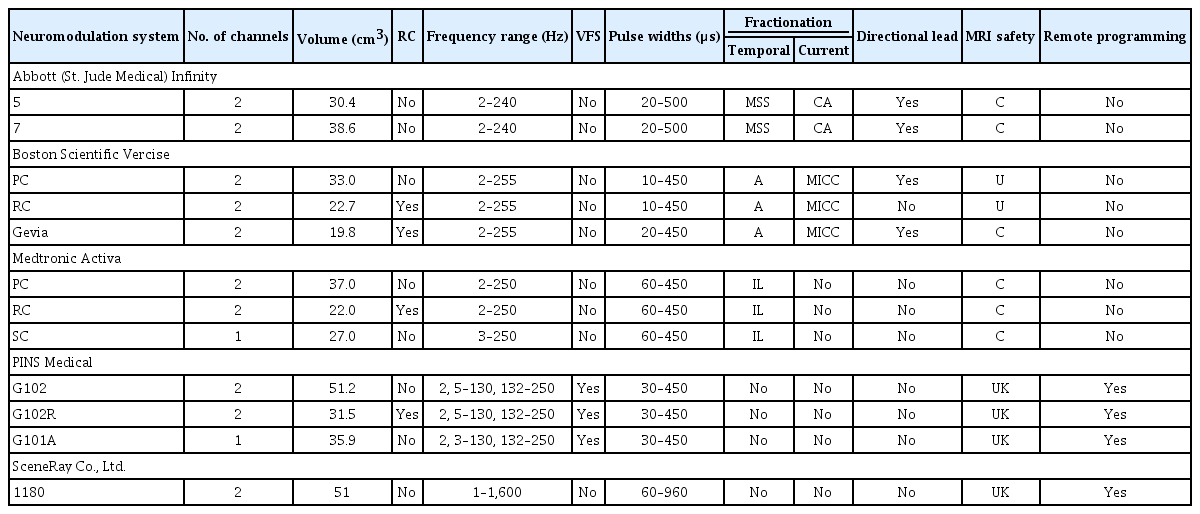
The Activa series is currently the most commonly implanted IPG. The Activa PC is smaller in size (volume of 37 cm3) and was proposed to have a longer battery life; however, studies have found a significant shorter life span for the Activa PC than for the Kinetra [60,61]. Subsequent additions to the Activa series included the rechargeable dual-channel Activa RC (22 cm3, approved in South Korea in 2010) and the single-channel nonrechargeable Activa SC (27 cm3). These IPGs can be used as either constant-voltage or constant-current devices. These devices are capable of interleaving stimulation, and patients who are implanted with these devices may receive full-body MRI when certain conditions are met (‘conditionally’ safe). The Activa PC and RC have a frequency range of 2–250 Hz, while the Activa SC has a frequency range of 3–250 Hz. Each device is capable of pulse widths ranging from 60–450 μs. These Medtronic devices are FDA approved for bilateral STN and GPi stimulation for PD, unilateral thalamic stimulation for ETs, and unilateral or bilateral stimulation of the GPi or STN for treatment of chronic, drug-refractory segmental or generalized dystonia. The same indications are approved in South Korea.
Abbott Infinity
The Abbott Infinity 5 and Infinity 7 IPGs are currently commercially available in the United States. Both models are constant-current, dual-channel, nonrechargeable devices. The infinity 5 IPG has a volume of 30.4 cm3 and has been designed for centers that utilize a single lead, similar to the Medtronic Activa SC. The Infinity 7 is a larger IPG designed for bilateral stimulation, with a volume of 38.6 cm3 and a predicted life span of 5 years. These devices have a frequency range of 2–240 Hz and pulse width range of 20–500 μs. Temporal and current fractionation is possible by means of multi-stim set and coactivation, respectively. Multi-stim set involves alternating stimulation of two separate contacts, and coactivation involves current fractionation to two separate contacts. Patients implanted with Infinity DBS systems can receive a full-body MRI when certain conditions are met. Currently, the Infinity system is FDA approved for bilateral STN and GPi stimulation for PD and for bilateral thalamic stimulation for ETs. In South Korea, these devices are also approved for dystonia.
Boston Scientific Vercise
Current commercially available IPGs include the Vercise PC, Vercise RC and Gevia. The Vercise PC is a constant-current, dual-channel, nonrechargeable device with a volume of 33 cm3. The Vercise RC is a rechargeable IPG with a volume of 22.7 cm3. The Vercise PC and RC are not MRI safe. The Gevia IPG is a dual-channel, rechargeable device with a volume of 19.8 cm3 that is MRI conditional. These devices have a frequency range of 2–255 Hz and a pulse width range of 20–450 μs. Temporal fractionation is accomplished using different ‘areas’ (2 per channel), and current fractionation may be accomplished via MICC independently per area. These systems are currently FDA approved for bilateral STN stimulation for PD. In South Korea, these devices are approved for PD, dystonia and medication-refractory tremors.
PINS Medical
The available IPGs manufactured by PINS Medical include the G102, G102R, and G102A. The G102 is a constant-voltage, nonrechargeable, dual-channel IPG similar to the Medtronic Activa PC with a volume of 51.2 cm3. The G102R is a dual-channel, rechargeable device with a volume of 31.5 cm3, and the G102A is a single-channel, nonrechargeable IPG with a volume of 35.9 cm3. The frequency range of these devices is 2–250 Hz, and the range of pulse widths is 30–450 μs. While these devices are not capable of current steering, similar to the Medtronic devices, they have been used for VFS and remote wireless programming. These devices are not FDA approved in the United States, but they currently are widely implanted throughout China.
SceneRay
The SceneRay model 1180 IPG is a constant-voltage, dual-channel, nonrechargeable IPG with a volume of 51 cm3. The frequency range of this device is 1–1,600 Hz, and the range of possible pulse widths is 60–960 μs. Similar to PINS Medical, use of wireless remote programming has been reported with this device [62].
The features of each of these devices are summarized in Table 1.
Commercially available DBS leads
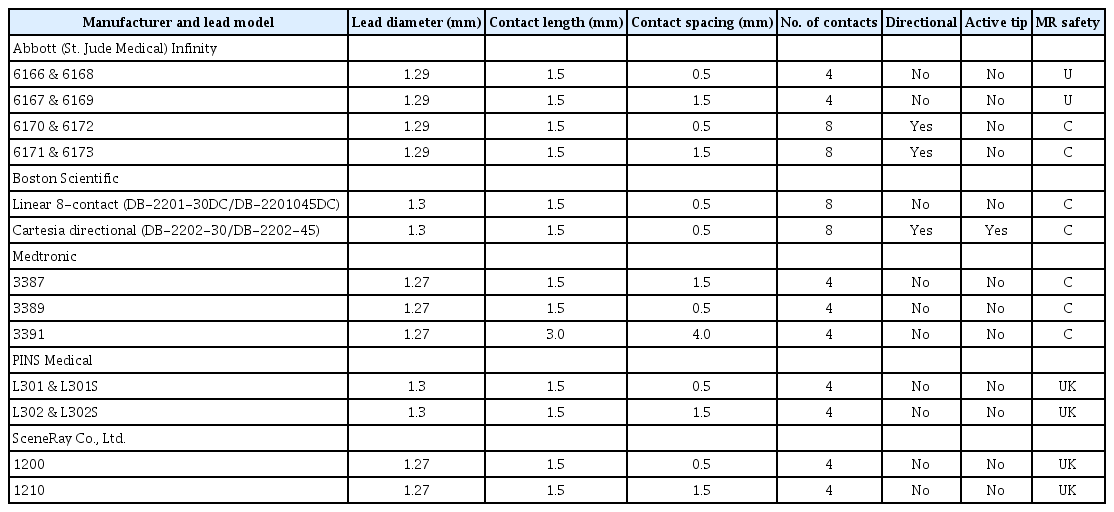
Medtronic
Current commercially available DBS leads from Medtronic include the models 3387, 3389, and 3391. Each lead is a traditional quadripolar DBS lead of 1.27 mm in diameter. The contacts of the 3387 and 3389 leads are 1.5 mm in length. The 3387 lead has a contact spacing of 1.5 mm, and the 3389 lead has a contact spacing of 0.5 mm. The 3391 DBS lead is specifically designed for targeting the anterior limb of the internal capsule for treatment of refractory obsessive-compulsive disorder. The contacts of the 3391 lead are 3 mm in length and separated from one another by 4 mm. The Medtronic DBS leads are MRI conditional.
Abbott Infinity
The Abbott DBS leads include both 4-contact quadripolar electrodes and 8-contact directional electrodes. Additionally, Abbott manufactures adaptors for Medtronic DBS electrodes that allow for compatibility with Abbott Infinity IPGs. The quadripolar leads for the Infinity system have a diameter of 1.29 mm and a total length of either 30 cm (models 6166 and 6167) or 40 cm (models 6168 and 6169). All have 4 contacts of 1.5 mm in length with spacing of either 0.5 mm (models 6166 and 6168) or 1.5 mm (models 6167 and 6169). These DBS leads are not MRI compatible. Similar to the quadripolar leads, Abbott directional leads have a diameter of 1.29 mm and come in lengths of 30 cm (models 6170 and 6171) or 40 cm (models 6172 and 6173). The center contacts, 2 and 3, consist of 3 radial contacts (named ‘a,’ ‘b,’ and ‘c;’ e.g., 2a) that allow for directional current steering, and contacts 1 and 4 are single contacts similar to those of the typical quadripolar leads. The directional leads include a radio-opaque marker proximal to contact 4 that can be used to determine the orientation of the directional contacts (direction of segment ‘a’). The contact spacing of models 6170 and 6172 is 0.5 mm, and it is 1.5 mm for models 6171 and 6173, making these leads ideal for directional stimulation in targets such as the GPi or the Vim. The Abbott directional DBS leads are MRI conditional.
Boston Scientific
Boston Scientific manufactures a linear, 8-contact lead as well as the Vercise Cartesia 8-contact directional lead, both of which have a diameter of 1.3 mm. Each lead type may have a length of 30 cm (models DB-2201-30DC and DB-2202-30) or 45 cm (DB-2201-45DC and DB-2202-45). Models DB-2201-30DC and DB-2201-45DC contain 8 contacts of 1.5 mm in length spaced by 0.5 mm. The Vercise Cartesia directional leads (models DB-2202-30 and DB-2202-45) contain two levels of 3 radially arranged directional contacts as well as a distal active tip and a proximal omnidirectional contact. The nonsegmented contacts are present at the bottom and top level of the lead (contact 1 and 8 for one channel and 9 and 16 for the other); segmented contacts are 2, 3, and 4 for the second level (10, 11, and 12 for the second channel), and 5, 6, and 7 are for the third level (13, 14, and 15 for the second channel). Similar to the Abbott directional lead, there is a proximal radio-opaque marker that enables determination of the orientation of the directional contacts (indicating the direction of the anterior contacts 2 and 5 or 10 and 13 for the other channel). All leads have a contact length of 1.5 mm and intercontact spacing of 0.5 mm. With a contact span of 15.5 mm, the linear 8-contact lead may be advantageous if a longer length of stimulation is required, such as when targeting the anterior limb of the internal capsule.
Similarly to Abbott, Boston Scientific manufactures adaptors for Medtronic DBS electrodes that allow for compatibility with their IPGs (23) [20].
PINS Medical
The DBS leads manufactured by PINS Medical closely resemble those of Medtronic. The models L302 and L302S are quadripolar leads with contacts of 1.5 mm in length separated from one another by 1.5 mm, similar to the 3397 Medtronic lead. Resembling the Medtronic 3389 lead, the model L301 and L301S leads include 1.5 mm contacts separated by 0.5 mm intervals. The PINS DBS leads have a diameter of 1.3. There is currently no available data to suggest that these leads are MRI conditional.
SceneRay
SceneRay currently offers three types of DBS electrodes, which differ in the spacing between contacts. All contacts are 1.5 mm in length, and each electrode is 1.27 mm in diameter. The model 1200 lead has 0.5 mm spacing between contacts, the model 1210 lead has 1.5 mm spacing, and the model 1211 has 1.0 mm spacing. Each type of lead is available in lengths of either 300 mm or 400 mm. One unique feature of the SceneRay DBS system is the TouchLoc lead anchoring device, which is an internationally patented lead fixture device that has been reported to result in less DBS lead drift and require significantly fewer adjustments than traditional lead anchors [63].
The features of each of the DBS leads described above are summarized in Table 2, and each electrode is visually presented in Figure 2.
CHOOSING THE BEST SYSTEM
Many factors may contribute to the choice of system, including the anatomical target, patient factors, and surgeon and neurologist preferences. The choice to use traditional quadripolar leads versus directional leads is most applicable when considering the size of the target nucleus and the potential for stimulation-related side effects. Conversely, patient factors are important when considering using a rechargeable versus a nonrechargeable IPG. While rechargeable devices are proposed to reduce the number of battery changes, sparing the patient repeated surgical procedures, patients must be reliable and capable of recharging their battery on a consistent basis. While the Medtronic Activa RC and Boston Scientific Gevia require charging only once weekly, patients who are disabled or have lack of social support may have difficulty assuming this responsibility. Additionally, the choice of DBS system relies heavily on the preferences and comfort of the treating surgeons and neurologists. Many practitioners have extensive experience using the Medtronic system since Medtronic has dominated the market for the past several decades. Patients living in remote regions or who are likely to relocate may find that Medtronic devices are more widely available.
Anatomical considerations
The STN is a small nucleus, measuring only 6-8 mm in its largest dimension. A typical trajectory of a DBS lead through the STN begins at the dorsolateral border and projects medially and ventrally while ideally avoiding the associative and limbic regions. Surrounding the nucleus laterally and, to some extent, anteriorly is the internal capsule. A lead positioned too lateral within the STN may produce contralateral face and limb contractions via current spread to internal capsule fibers. A lead that is positioned too medial may stimulate fibers of the adjacent oculomotor tract, resulting in eye deviation and diplopia. Additionally, there have been reports of stimulation of ventrally located contacts causing acute hypomanic episodes [64] as well as stimulation of the underlying substantia nigra resulting in hypomania and depression [65,66]. Anteriorly positioned leads may project into the lateral hypothalamic region, leading to stimulation-related autonomic effects, such sweating and nausea.
The GPi, in contrast to the STN, is a larger target associated with a lower incidence of side effects. The internal capsule borders the GPi anteriorly and medially, and the optic tract is located ventrally. Also located ventral to the GPi is the ansa lenticularis, which separates the GPi from the nucleus basalis of Meynert and the amygdala. A typical DBS lead trajectory extends along the posterolateral aspect of the GPi and just dorsal to the optic tract. While side effects are generally rare, medial or posterior placement of the DBS lead can result in capsular stimulation and contralateral face and limb contractions, and ventral deviation can produce phosphene and visual phenomena.
The potential for stimulation-related side effects with STN-DBS may warrant consideration of directional DBS leads. One technical point to consider when implanting directional leads is that the second and third levels of contacts contain the directional contacts such that the lead may need to be implanted deeper than usual in order to place the directional contacts within the target region.
Another potential strategy for increasing the therapeutic window for stimulation while avoiding stimulation-induced side effects is the use of shorter pulse widths < 60 μs. Experimental studies in animals have demonstrated that axons are more excitable than cell bodies [18], and the threshold for activation of neural elements varies with stimulus strength and pulse width. Longer pulse widths may facilitate stimulation of larger diameter, myelinated axons, such as those arising from pyramidal tracts, and shorter pulse widths are believed to target current to cell bodies and smaller axons. Reich et al. [19] observed an inverse relationship between pulse width duration and the threshold for side effects in a study group of four patients with STN-DBS. The authors implemented the Boston Scientific Vercise neurostimulation system for delivery of pulse widths < 60 μs and observed a twofold increase in the therapeutic window of stimulation with a pulse width of 30 μs compared with the standard pulse width of 60 μs. Currently, both the Boston Scientific Vercise and Abbott Infinity systems allow for pulse widths shorter than 60 μs.
Rechargeable vs. nonrechargeable IPGs
The most common nonrechargeable IPG, the Medtronic Activa PC, has an average battery lifespan ranging from 2.6 to 4.5 years [60,61,67,68], which is approximately 2.5 years shorter than its predecessor, the Kinetra [61]. This is considerably shorter than the marketed lifespan of the PC, which is advertised as approximately 5 years. The lifespan of an IPG is largely dependent on the current demands of the patient’s stimulation settings, with high-voltage stimulation necessitating more frequent battery changes. Rechargeable IPGs were first introduced in 2008, and the predicted lifespan of rechargeable IPGs according the manufacturers ranges from 10 for Brio (Abbott/St. Jude) to 15 (Medtronic), up to 25 (Boston Scientific) years, which has yet to be confirmed.
The goal of rechargeable IPGs is to reduce the number of battery changes, which may reduce costs associated with multiple surgical procedures and minimize the chances of hardware infections. Some studies have reported an increased incidence of infections with repeated IPG replacements [69,70], but one study did not find such an association [71]. Nevertheless, the use of a rechargeable IPG over a 9-year period (the initially approved lifespan of Activa RC) has been estimated to lead to savings of $60,900 based on a single-center study involving 206 patients [72]. Since rechargeable IPGs became available, many patients have been switched to rechargeable devices, and some have even received rechargeable IPGs during initial DBS implantation [72,73]. Patient surveys have demonstrated that the vast majority of patients find the recharging process to be easy, and the incidence of adverse events and temporary interruptions in therapy is low [67,72,73].
The decision to use a rechargeable versus a nonrechargeable IPG is generally left to the discretion of the treating physician and may be influenced by the current requirements of the stimulation settings as well as the reliability of the patient to recharge their device (Table 3). The Multi-Recharge Trial, conducted at four neurosurgical centers across Germany, reported on the survey results of 56 patients switched to rechargeable IPGs and 139 patients who were implanted with rechargeable IPGs during DBS implantation [67]. According to survey results, patients charged their IPGs once every 10 ± 8 days and spent a mean of 122 ± 175 minutes per week recharging their device. Dystonia patients found the recharging process to be significantly more convenient than PD patients. Interestingly, the Activa RC was found to require significantly more charging time per week than the Boston Scientific Vercise and Abbott (St. Jude) Brio. While charging failures were uncommon, they were more likely to occur with patients who were switched from a nonrechargeable IPG. While the decision to use a rechargeable IPG should take patient factors into consideration, the results of these survey studies suggest that rechargeable IPGs are effective and easy to manage even in older patients [67,73].
CONCLUSIONS
Technological advances in DBS hardware and software presently comprise the greatest area of growth within the field of functional neurosurgery. While studies evaluating the features of new DBS systems have found gains in measures of effective stimulation, further research is needed to characterize the impact of these technologies on patient outcomes and quality of life. In a small-sample, cross-sectional, questionnaire-based study, it was found that patients treated with an established DBS system (Medtronic) were less inclined to think that additional programming would lead to further improvement, thus suggesting that the additional features of newer devices may complicate the DBS programming of patients, increasing the number of visits necessary to convince patients that stimulation is optimized and thus possibly increasing physicians’ and patients’ frustrations [74].
Many of the features of these new systems currently focus on minimizing stimulation-related side effects. Further understanding of the pathophysiology of these disorders—and PD in particular—is required to determine if DBS therapy is capable of addressing symptoms refractory to standard medications or DBS, such as postural instability, ataxia and FOG. Exploration of other surgical targets, such as the substantial nigra pars reticulata [75,76] and pedunculopontine nucleus [77,78], have sought to address levodopa-resistant FOG in PD. With continued research, deeper insights into the neural networks that contribute to these disorders in synergy with advancements in stimulation technology will hopefully yield new therapies that can improve the quality of life of these patients.
Notes
Conflicts of Interest
AML has received honoraria from Abbott, Boston Scientific, and Medtronic. AF has received honoraria from Abbott, Boston Scientific, Brainlab and Medtronic.
Author Contributions
Conceptualization: Alfonso Fasano. Data curation: Michelle Paff. Methodology: Michelle Paff. Supervision: Andres M. Lozano, Alfonso Fasano. Visualization: Aaron Loh. Writing—original draft: Michelle Paff, Aaron Loh, Can Sarcia. Writing—review & editing: Andres M. Lozano, Alfonso Fasano. Approval of final manuscript: all authors.
Acknowledgements
Authors are grateful to Desiree Burdyshaw, Binith Cheeran (Abbott) and Benjamin Nagy (Boston Scientific) for having provided some technical information about the devices produced by their companies.