Articles
- Page Path
- HOME > J Mov Disord > Volume 15(1); 2022 > Article
-
Brief communication
Dance Intervention Using the Feldenkrais Method Improves Motor, and Non-Motor Symptoms and Gait in Parkinson’s Disease: A 12-Month Study -
Sung Hoon Kang1*
 , Jinhee Kim1*
, Jinhee Kim1* , Ilsoo Kim1
, Ilsoo Kim1 , Young Ae Moon2, Sojung Park2
, Young Ae Moon2, Sojung Park2 , Seong-Beom Koh1
, Seong-Beom Koh1
-
Journal of Movement Disorders 2022;15(1):53-57.
DOI: https://doi.org/10.14802/jmd.21086
Published online: November 3, 2021
1Department of Neurology, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
2Representative Feldenkrais Training Institute of Korea, Seoul, Korea
- Corresponding author: Seong-Beom Koh, MD, PhD Department of Neurology, Korea University Guro Hospital, Korea University College of Medicine, 148 Gurodong-ro, Guro-gu, Seoul 08308, Korea / Tel: +82-2-2626-3169 / Fax: +82-2-2626-1257 / E-mail: parkinson@korea.ac.kr
- *This authors contributed equally to this work.
Copyright © 2022 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objective
- The aim of this study was to assess the effects of dancing (using the Feldenkrais method) on motor and non-motor symptoms, quality of life (QoL), and objective parameters of gait at the time of intervention and at the end of the 1-year study period.
-
Methods
- This was a single-arm study in which 12 subjects with Parkinson’s disease (PD) received dance intervention during a 6-month period. Objective motor scales, gait analysis, and questionnaires on non-motor symptoms were evaluated at baseline and at 3, 6, and 12 months.
-
Results
- Dance intervention decreased motor scale (Unified Parkinson’s Disease Rating Scale and Tinetti scale) scores and improved gait disturbance (gait velocity and step length) without increasing levodopa equivalent dose. Furthermore, dancing decreased non-motor scale (Non-Motor Symptoms Scale and Montgomery-Asberg Depression Rating Scale) scores and improved QoL.
-
Conclusion
- Our findings suggest that dance intervention can be a complementary management method for PD patients.
- Study design and participants
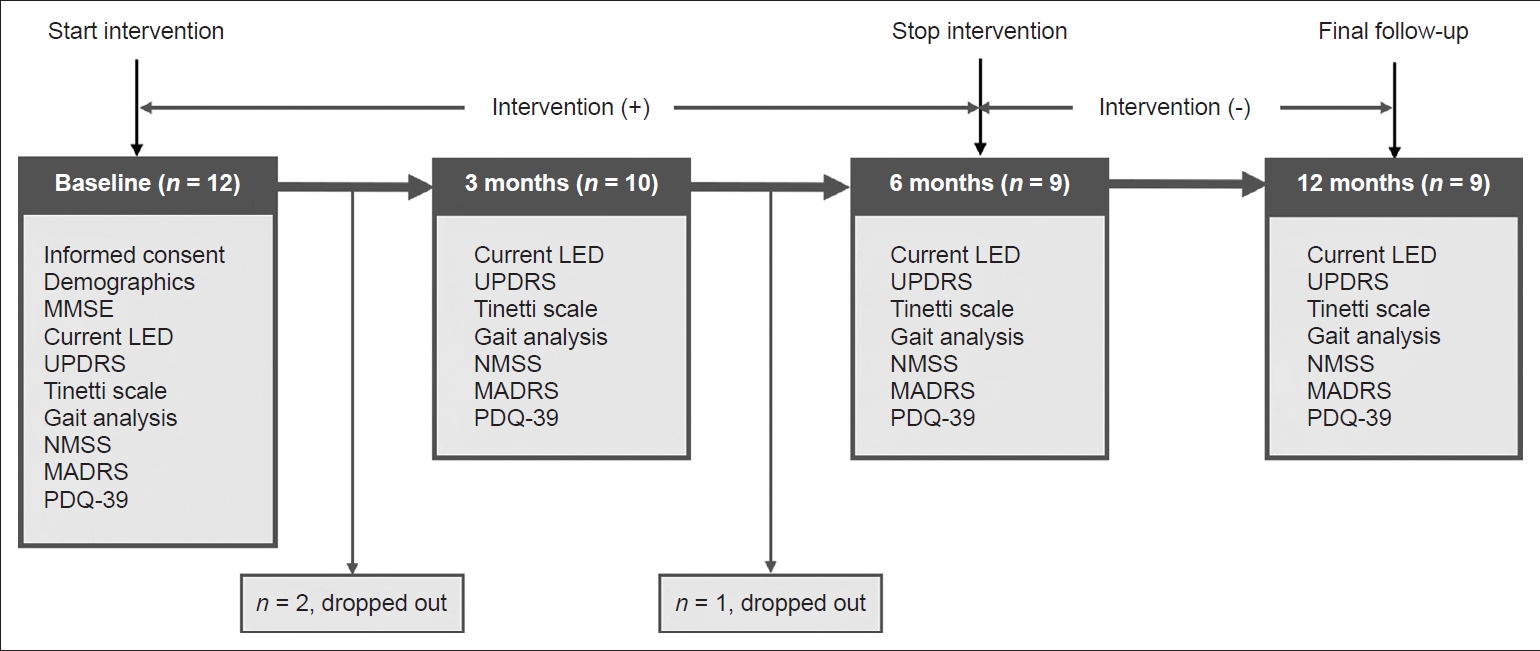
- The present study was a 1-year, single-center, single-arm study of patients with PD. From June 2019 to July 2019, 12 prospectively recruited subjects with PD who visited the movement disorder clinic of Korea University Guro Hospital in Seoul, Korea, were recruited. Detailed inclusion and exclusion criteria are presented in Supplementary Material 1 (in the online-only Data Supplement). After recruitment, subjects received a dance intervention based on the Feldenkrais method [5] in addition to classical dopaminergic medications (Supplementary Material 2 in the online-only Data Supplement). The intervention was performed once a week over a 6-month period. Baseline comprehensive motor function evaluations, including the Unified Parkinson’s Disease Rating Scale (UPDRS) [6], Hoehn and Yahr stage [7], and Tinetti scale [8,9]; gait analysis; and questionnaires on non-motor symptoms, including the Non-Motor Symptoms Scale (NMSS) [10], Montgomery-Asberg Depression Rating Scale (MADRS) [11], and Parkinson’s Disease Questionnaire-39 (PDQ-39) [12], were completed for all subjects (Supplementary Material 3 in the online-only Data Supplement). Follow-up motor function evaluation, gait analysis, and questionnaires were performed after 3 and 6 months. The dance intervention was terminated at six months, and then subjects only received classical dopaminergic medications. The final follow-up motor function evaluation, gait analysis, and questionnaires were completed at 12 months (6 months after discontinuation of dance intervention). Three subjects did not attend more than 50% of the dance intervention and were excluded (Figure 1). Because subjects who dropped out during follow-up were unavailable for follow-up assessment, 9 subjects were included in the final analyses.
- This study was approved by the Institutional Review Board of Korea University Guro Hospital (IRB No. 2019GR0023). Written informed consent was obtained from the patients.
- Statistical analyses
- To explore the effects of dancing on motor and non-motor symptoms in patients with PD, the Wilcoxon signed rank test was used for the UPDRS part III (UPDRS III), Tinetti scale, NMSS, MADRS, and PDQ-39 scores at baseline and at 3, 6, and 12 months. To investigate the effects of dancing on gait function, the Wilcoxon signed rank test was used for the parameters of gait velocity, cadence, step length, and step length covariance at baseline and at 3, 6, and 12 months.
- All reported p-values were two-sided, and the statistical significance level was set at 0.05. All analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA).
MATERIALS & METHODS
- Patient demographics
- The mean baseline age was 69.1 ± 4.3 years, and 4 of 9 (44.4%) patients were female. The mean duration to disease onset was 5.3 ± 3.7 years. The mean UPDRS III, Tinetti scale, NMSS, MADRS, and PDQ-39 scores at baseline were 18.2 ± 6.5, 24.8 ± 1.2, 29.7 ± 20.6, 10.3 ± 8.3, and 21.4 ± 15.5, respectively (Supplementary Table 1 in the online-only Data Supplement).
- Effects of dancing on longitudinal motor symptoms
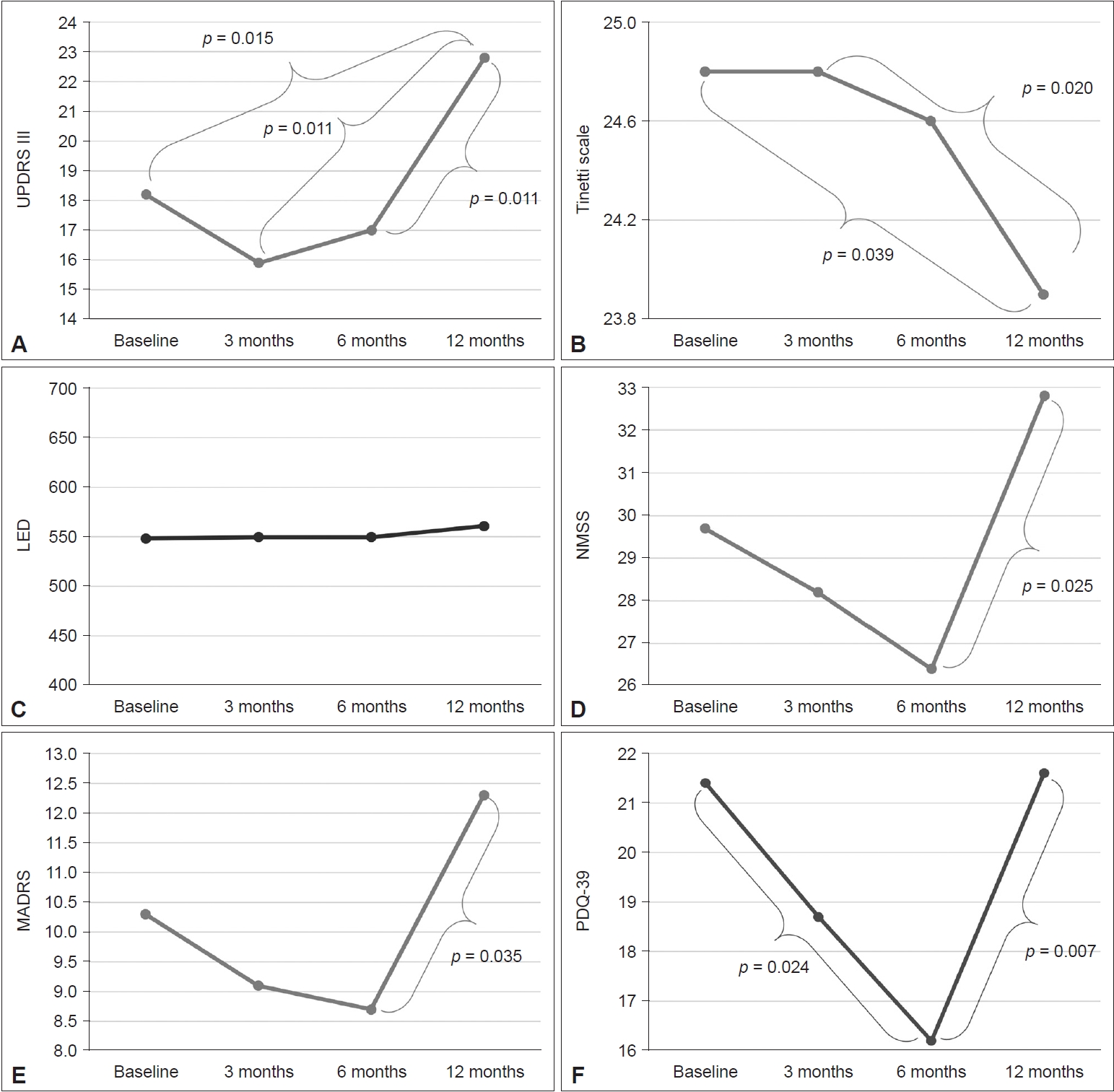
- During the dance intervention, UPDRS III scores at 3 and 6 months were lower than at baseline, although without statistical significance (baseline vs. 3 months, p = 0.092; baseline vs. 6 months, p = 0.438). After the dance intervention, the UPDRS III score at 12 months was significantly higher than that at baseline and at 3 and 6 months (baseline vs. 12 months, p = 0.015; 3 months vs. 12 months, p = 0.011; 6 months vs. 12 months, p = 0.011) (Figure 2A). Tinetti scale scores at baseline and at 3 and 6 months did not differ (baseline vs. 3 months, p = 1.000; baseline vs. 6 months, p = 0.581; 3 months vs. 6 months, p = 0.458); however, Tinetti scale scores at 12 months were lower than at baseline and at 3 months (baseline vs. 12 months, p = 0.039; 3 months vs. 12 months, p = 0.020) (Figure 2B). Furthermore, during the dance intervention, the levodopa equivalent dose (LED) was stable over 6 months; however, the LED was increased at 12 months after the dance intervention (Figure 2C).
- Effects of dancing on longitudinal gait disturbance
- Gait velocity (3 months vs. 6 months, p = 0.028) and step length (3 months vs. 6 months, p = 0.011) improved between 3 and 6 months. After dance intervention, gait velocity and step length worsened (Supplementary Figure 1A and C in the online-only Data Supplement). Cadence was stable for 6 months during the dancing intervention but increased at 12 months after the dance intervention (Supplementary Figure 1B in the online-only Data Supplement). However, cadence was not significantly different at baseline vs. 3 months (p = 0.678), 3 months vs. 6 months (p = 0.859), and 6 months vs. 12 months (p = 0.192). Step length covariance decreased over the 6-month period but increased at 12 months; however, the changes were not statistically significant during follow-up (Supplementary Figure 1D in the online-only Data Supplement).
- Effects of dancing on longitudinal non-motor symptoms
- During the dancing intervention, NMSS scores decreased steadily from baseline to 3 and 6 months, although without statistical significance (baseline vs. 3 months, p = 0.859; baseline vs. 6 months, p = 0.312; 3 months vs. 6 months, p = 0.260). After the dance intervention, the NMSS score at 12 months was significantly higher than that at 6 months (6 months vs. 12 months, p = 0.025) (Figure 2D). The MADRS score decreased steadily from baseline to 3 and 6 months, although without statistical significance (baseline vs. 3 months, p = 0.234; baseline vs. 6 months, p = 0.154; 3 months vs. 6 months, p = 0.766). After dance intervention, the MADRS score at 12 months was significantly higher than that at 6 months (6 months vs. 12 months, p = 0.035) (Figure 2E). The PDQ-39 score decreased steadily from baseline to 3 and 6 months, and the scores at 6 months were significantly lower than those at baseline (p = 0.024). However, because the score increased after the dance intervention, the PDQ-39 scores at 12 months were higher than those at 6 months (p = 0.007) (Figure 2F).
RESULTS
- In the present study, the effects of dancing on motor or non-motor symptoms in patients with PD were investigated. The major findings showed that dancing decreased motor scale scores and improved gait disturbance without increasing LED, decreased the non-motor scale scores, and improved QoL. Taken together, the results indicated that dancing can be an alternative therapy for the management of various symptoms in PD.
- As expected, dancing decreased the motor scale (UPDRS III and Tinetti scale) scores and improved gait disturbance. Specifically, the UPDRS III score, representing the severity of motor symptoms in PD, decreased over the first 6 months when subjects received the dancing intervention despite stable LED. However, after discontinuing dance intervention, the UPDRS III score sharply increased at 12 months. The Tinetti scale score, which represents the severity of imbalance and gait disturbance, showed a similar pattern to the UPDRS III score. Although it was not possible to compare the progression of motor symptoms between groups with and without dance intervention due to the study design, when considering the features of degenerative disease, dancing might have positive effects on motor symptoms in PD. In addition, gait parameters of velocity, step length, and step length covariance improved during dance intervention. To the best of our knowledge, this was the first report in which the relationship between dancing (based on the Feldenkrais method) and objective motor scale scores in patients with PD was presented. In terms of non-motor symptoms, dancing decreased the non-motor scale (NMSS and MADRS) scores and improved QoL (PDQ-39). These findings were consistent with the results in a previous study showing that the Feldenkrais method could affect depressive symptoms and the QoL of patients with PD [13].
- The present study had several limitations. First, the sample size was relatively small because this was a pilot study. Second, the effects of dancing on motor or non-motor symptoms were modest. In particular, a multiple comparisons correction, which might lead to Type I error, was not performed. However, because this was an exploratory study, the multiple comparison correction might result in overlooking the important associations shown in the preliminary analysis. Further studies using large sample size and randomized controlled trial design are needed to confirm our results. Nevertheless, the study results are noteworthy because this is the first report showing the effects of dancing on objective motor scale scores in patients with PD. Because frequent falls due to imbalance and gait disturbance are not well controlled with medical treatment and closely associated with poor prognosis and mortality in patients with PD, the findings indicate that dance intervention might be a complementary management for PD, although further studies are needed to support the results.
DISCUSSION
Supplementary Materials
Supplementary Figure 1.
-
Conflicts of Interest
The authors have no financial conflicts of interest.
-
Funding
None.
-
Author Contributions
Conceptualization: Seong-Beom Koh. Data curation: Jinhee Kim, Ilsoo Kim, Young Ae Moon, Sojung Park. Formal analysis: Sung Hoon Kang, Jinhee Investigation: Sung Hoon Kang, Jinhee Kim. Methodology: Jinhee Kim, Seong-Beom Koh. Project administration: Seong-Beom Koh. Supervision: Seong-Beom Koh. Visualization: Sung Hoon Kang. Writing—original draft: Sung Hoon Kang. Writing—review & editing: Sung Hoon Kang, Seong-Beom Koh.
Notes


- 1. Rahman S, Griffin HJ, Quinn NP, Jahanshahi M. Quality of life in Parkinson’s disease: the relative importance of the symptoms. Mov Disord 2008;23:1428–1434.ArticlePubMed
- 2. Alves Da Rocha P, McClelland J, Morris ME. Complementary physical therapies for movement disorders in Parkinson’s disease: a systematic review. Eur J Phys Rehabil Med 2015;51:693–704.PubMed
- 3. Buchanan PA. A preliminary survey of the practice patterns of United States Guild Certified Feldenkrais PractitionersCM. BMC Complement Altern Med 2010;10:12.ArticlePubMedPMC
- 4. Hillier S, Worley A. The effectiveness of the Feldenkrais Method: a systematic review of the evidence. Evid Based Complement Alternat Med 2015;2015:752160.ArticlePubMedPMC
- 5. Batson G, Deutsch JE. Effects of Feldenkrais awareness through movement on balance in adults with chronic neurological deficits following stroke: a preliminary study. Complement Health Pract Rev 2005;10:203–210.Article
- 6. Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord 2003;18:738–750.ArticlePubMed
- 7. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–442.ArticlePubMed
- 8. Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc 1986;34:119–126.ArticlePubMed
- 9. Park J, Koh SB, Kim HJ, Oh E, Kim JS, Yun JY, et al. Validity and reliability study of the Korean Tinetti Mobility Test for Parkinson’s disease. J Mov Disord 2018;11:24–29.ArticlePubMedPMC
- 10. Koh SB, Kim JW, Ma HI, Ahn TB, Cho JW, Lee PH, et al. Validation of the Korean-version of the non-motor symptoms scale for Parkinson’s disease. J Clin Neurol 2012;8:276–283.ArticlePubMedPMC
- 11. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382–389.ArticlePubMed
- 12. Kwon DY, Kim JW, Ma HI, Ahn TB, Cho J, Lee PH, et al. Translation and validation of the Korean version of the 39-item Parkinson’s disease questionnaire. J Clin Neurol 2013;9:26–31.ArticlePubMedPMC
- 13. Teixeira-Machado L, Araújo FM, Cunha FA, Menezes M, Menezes T, Melo DeSantana J. Feldenkrais method-based exercise improves quality of life in individuals with Parkinson’s disease: a controlled, randomized clinical trial. Altern Ther Health Med 2015;21:8–14.
REFERENCES
Figure & Data
References
Citations

- Mild cognitive impairment is associated with poor gait performance in patients with Parkinson’s disease
Sung Hoon Kang, Jinhee Kim, Jungyeun Lee, Seong-Beom Koh
Frontiers in Aging Neuroscience.2022;[Epub] CrossRef
Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite