Articles
- Page Path
- HOME > J Mov Disord > Volume 8(2); 2015 > Article
-
Letter to the editor
Two Cases of Secondary Hemifacial Spasm: Pathophysiology and Management - Frederick A. Zeiler, Anthony M. Kaufmann
-
Journal of Movement Disorders 2015;8(2):103-105.
DOI: https://doi.org/10.14802/jmd.15004
Published online: May 31, 2015
Section of Neurosurgery, Department of Surgery, University of Manitoba, Winnipeg, Manitoba, Canada
- Corresponding author: Frederick A. Zeiler, MD, Section of Neurosurgery, Department of Surgery, University of Manitoba, Health Sciences Center, GB-1 820 Sherbrook Street, Winnipeg, Manitoba R3A1R9, Canada / Tel: +12042286623 / Fax: +12047873851 / E-mail: umzeiler@cc.umanitoba.ca
• Received: January 27, 2015 • Revised: February 14, 2015 • Accepted: March 25, 2015
Copyright © 2015 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- A 56-year-old female with a known left sided vestibular schwannoma (VS) that was treated with fractionated stereotactic radiotherapy 5 years prior, presented with a 12 month history of acute onset and progressive HFS, characterized by periorbital twitching. At the time of radiosurgery the tumor measured 2.5 cm in diameter. Post treatment, she developed gradual sensory neural hearing loss, leaving her with a 50% reduction in hearing in the left ear. Brain magnetic resonance imaging (MRI) displayed no change in size since radiosurgery. It was elected to undertake a surgical debulking of the tumor combined with microvascular decompression (MVD) for HFS.
- Intra-operatively a left retro-sigmoid approach to the CPA was taken. The inferior margin of the tumor was then exposed at the level of the pontomedullary sulcus, identifying an ectatic vertebral artery and the left posterior inferior cerebellar artery (PICA) grooved into the caudal pons at the region of the root exit zone (REZ) of the facial nerve. The PICA was then elevated and mobilized from the REZ of the facial nerve and shredded Teflon felt was placed to maintain the vessel away from the nerve. Internal debulking of the tumor was performed, and the facial nerve was dissected off of the tumor capsule.
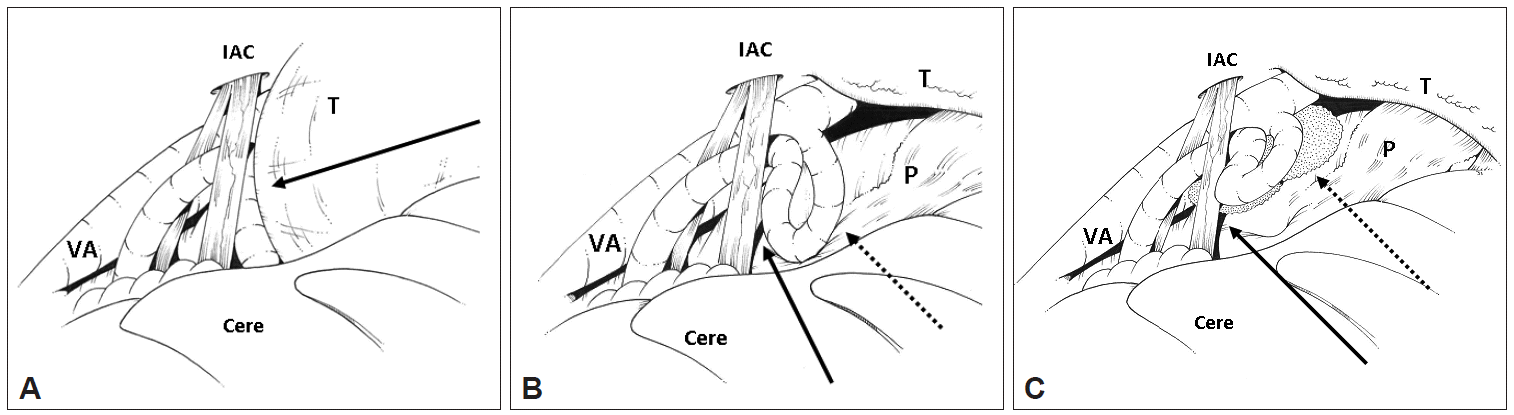
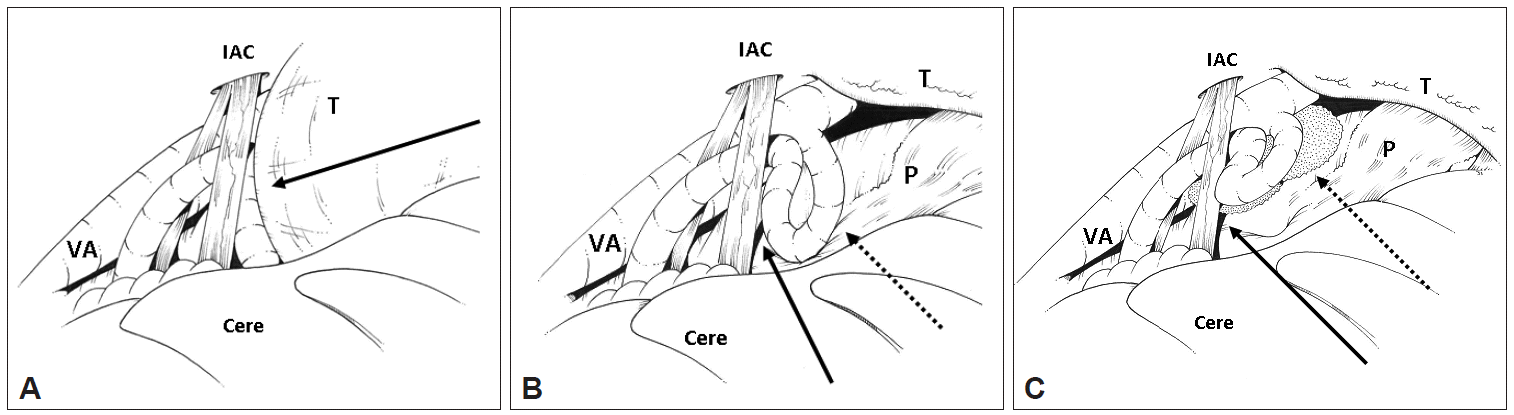
- The bone overlying the internal acoustic canal (IAC) was removed exposing the dura of the IAC, which was then incised allowing tumor to spontaneously herniated through the opening. This decompressed the intra-cannulicular portion of the facial nerve. Intra-operative monitoring demonstrated electromyographic lateral spread that was successfully abolished with the decompression. Brainstem auditory evoked potentials (BAEP) remained stable. Intra-operative illustrations of the lesion and MVD of the facial nerve can be seen in Figure 1.
- Post-operatively her HFS improved and eventually abated, and hearing remained unchanged. She encountered no postoperative complications. She remains spasm free since 2003. The final pathology of the tumor returned as VS.
Case 1
- A 51-year-old male, actor by profession, presented with a history of 3 episodes of right tonic facial contraction lasting several minutes. Further investigation he revealed decreased hearing in the right ear over the last 12 months and subtle balance difficulties. Brain MRI demonstrated a right CPA angle tumor filling the IAC, with mild compression of the middle cerebellar peduncle.
- It was elected to perform a surgical debulking of the tumor and facial nerve decompression. Complete tumor resection was not planned, in order to minimize risks of cranial nerve injury.
- A right retro-sigmoid approach was taken, with the origin of the facial nerve identified below the lower pole of the tumor, as confirmed with stimulation. There was no evidence of NVC on the REZ of the facial nerve. The lateral surface of the tumor was noted to be free of vessels and nerves, it was subsequently incised and internal debulking of the tumor occurred. Debulking stopped once tumor attached to the vestibule-cochlear nerve was noted to be adherent.
- The IAC was opened as described in case 1 causing the tumor to herniate through the opening. This decompressed the intra-cannulicular portion of the facial nerve. Dissection of the tumor off of the facial nerve was not conducted given adherence. Facial nerve electromyography did not display any injury potentials. The BAEP could not be obtained at the onset of surgery.
- Post-operatively the tonic facial contractions completely resolved and there were no complications. Pathology returned as a World Health Organization grade I meningioma. Residual tumor will be followed radiographically with a plan for radiosurgery if growth is demonstrated.
Case 2
- A few important points can be taken from these cases. First, the atypical clinical presentation (case 2) highlights the difference between classic primary HFS (case 1), related to pulsatile vascular compression of the facial nerve, and compression secondary to a CPA/IAC tumor. Second, the potential reversible nature of the primary or secondary HFS related to tumor CPA tumors has been displayed in both cases. For the classical HFS symptoms (case 1), alleviation of NVC at the REZ of the facial nerve was effective while the tonic facial contractions of secondary HFS (case 2) were successful alleviated with tumor decompression, particularly in the IAC. As a result, when presented with CPA tumors and facial twiching one must consider whether extensive tumor debulking versus alleviation of potential NVC is warranted. Symptoms of HFS secondary to NVC should be treated via standard MVD techniques, with elevation of the culprit vessel from the facial nerve and maintenance of separation with Teflon felt. Fourth, we do not believe that radiosurgery would be effective to treat either of these conditions, and was in fact utilized previously in our case 1. Smaller CPA tumors such as those found in our 2 cases are now often treated with stereotactic radiosurgery (SRS). This offers excellent tumor growth control and often regression in size over a couple of years. There is some data in the literature to suggest that SRS for tumor related HFS may lead to symptom resolution [8], with a latency period of 12 months or more until symptom improvement. In cases such as presented here, however, the most prominent clinical manifestations were HFS and best treated with decompression of the relevant point of symptomatic compression. In our case 1 where SRS was previously utilized, the patient had 5 years of follow-up and 12 months of progressive HFS symptoms. Given previous SRS and a lack of change in tumor on MRI, we suspected NVC of the facial nerve and elected for surgical exploration rather than repeat SRS. Finally, complete surgical resection of these tumors was not necessary to achieve resolution of symptoms. Only decompression and alleviation of the associated NVC were required. This concept is important, as aggressive surgical removal of these tumors is associated with a higher risk of facial and vestibular-cochlear nerve impairment.
Discussion
- We would like to express our thanks to Jon Stepaniuk for the professional illustrations provided for the operative technique described.
Acknowledgments
Figure 1.Intra-operative illustration of tumor and vascular compression. A: Lateral view of the cerebellopontine angle via a retro-sigmoid approach. Cerebellum retracted gently to expose the T compressing the posterior inferior cerebellar artery (PICA) (solid black arrow) and displacing it towards the cranial nerve (CN) VII and VII complex. B: T has been debulked, exposing the P and the loop of PICA contacting both the pons near the facial root entry zone (dotted arrow) and CN VII (solid arrow). C: PICA has been mobilized from the P and CN VII and padded with Teflon felt at both sites (dotted and solid arrows). IAC: internal acoustic canal, VA: vertebral artery, T: tumor, Cere: cerebellum, P: pons.

- 1. Campos-Benitez M, Kaufmann AM. Neurovascular compression findings in hemifacial spasm. J Neurosurg 2008;109:416–420.ArticlePubMed
- 2. Jannetta PJ. Arterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgia. J Neurosurg 1967;26:Suppl:159–162.Article
- 3. Barker FG 2nd, Jannetta PJ, Bissonette DJ, Shields PT, Larkins MV, Jho HD. Microvascular decompression for hemifacial spasm. J Neurosurg 1995;82:201–210.ArticlePubMed
- 4. Han IB, Chang JH, Chang JW, Huh R, Chung SS. Unusual causes and presentations of hemifacial spasm. Neurosurgery 2009;65:130–137.discussion 137. ArticlePubMedPDF
- 5. Yaltho TC, Jankovic J. The many faces of hemifacial spasm:differential diagnosis of unilateral facial spasms. Mov Disord 2011;26:1582–1592.ArticlePubMed
- 6. Lee SH, Rhee BA, Choi SK, Koh JS, Lim YJ. Cerebellopontine angle tumors causing hemifacial spasm: types, incidence, and mechanism in nine reported cases and literature review. Acta Neurochir (Wien) 2010;152:1901–1908.ArticlePubMed
- 7. Barker FG 2nd, Jannetta PJ, Babu RP, Pomonis S, Bissonette DJ, Jho HD. Long-term outcome after operation for trigeminal neuralgia in patients with posterior fossa tumors. J Neurosurg 1996;84:818–825.ArticlePubMed
- 8. Chang CS, Chuang CC, Wu MF, Liu WS, Tu HT, Huang CF. Gamma Knife surgery for hemifacial spasm related to cerebellopontine angle tumors. J Neurosurg 2012;117 Suppl:170–174.ArticlePubMed
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- A Case Report of Hemifacial Spasm Caused by Vestibular Schwannoma and Literature Review
Xiaomin Cai, Yinda Tang, Hua Zhao, Zheng Chen, Haopeng Wang, Wanchun Zhu, Shiting Li
Brain Sciences.2022; 12(10): 1347. CrossRef
Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite