Articles
- Page Path
- HOME > J Mov Disord > Volume 8(3); 2015 > Article
-
Review Article
The Current Status of Deep Brain Stimulation for the Treatment of Parkinson Disease in the Republic of Korea - Jung-Il Lee
-
Journal of Movement Disorders 2015;8(3):115-121.
DOI: https://doi.org/10.14802/jmd.15043
Published online: September 10, 2015
Department of Neurosurgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- Corresponding author: Jung-Il Lee, MD, Department of Neurosurgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, Korea Tel: +82-2-3410-3494 Fax: +82-2-3410-0048 E-mail: jilee@skku.edu
• Received: July 7, 2015 • Revised: July 22, 2015 • Accepted: July 28, 2015
Copyright © 2015 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Parkinson disease (PD) is a common neurodegenerative disease with an increasing prevalence in Korea. Deep brain stimulation (DBS) is a safe and effective surgical treatment option for this disease. The aim of this review was to provide an update regarding current DBS practices with respect to the treatment of PD in the Republic of Korea. The first DBS in Korea was performed in 2000; approximately 2,000 patients have undergone DBS for a variety of neurological disorders, the majority of whom were patients with PD. Approximately 150 new patients with PD receive DBS annually, and more than 20 centers perform DBS. However, DBS remains underutilized for many reasons, and the clinical case burden at many institutions is below the level presumed adequate for qualified practice. With a rapidly aging population and an evolving socioeconomic environment, the need for surgical intervention for PD is likely to increase significantly in the future. Many issues such as finances, education, and quality assurance must be resolved to cope with this need.
- Parkinson disease (PD) is a progressive, neurodegenerative disorder that results in cardinal motor symptoms such as tremor, rigidity, and bradykinesia, as well as postural and gait disturbances. PD is also associated with significant non-motor symptoms, which are linked to either primary disease or therapies used in its management. Many of the cardinal symptoms of PD may be attenuated by pharmacological treatment; however, long-term treatment is associated with motor fluctuations and non-motor and motor complications. Since the 1980s, deep brain stimulation (DBS) has evolved as a well-established surgical option for patients suffering from advanced stage PD.
- The prevalence and incidence of PD vary considerably due to a lack of reliable statistics derived from large scale population studies using definitive diagnostic tools. Epidemiological data for PD in Korea are scarce. In a publication based on the statistical yearbook of Health Insurance in Korea, the prevalence of PD in the year 2000 was estimated between 54 and 68/100,000 people, or approximately 30,000 patients and 3,000 new patients every year [1]. In an epidemiological study published in 2007, the prevalence of PD in Korea was reported as 1.47% of the population older than 60 years of age [2]. The crude prevalence of PD estimated in another study using dopamine transporter imaging was 0.42 per 100 persons (aged 65 years or older) [3] and indicated that there exists great variability regarding the estimates of the prevalence of PD. Certainly the number of patients with PD in Korea will rapidly increase as Korea’s population ages. Based on database of Statistics Korea, the population of individuals over 65 years of age was 5.45 million in 2010 and will likely increase to 12.69 million in 2030 [4]. Additionally, the mean duration of the illness among affected individuals will increase, resulting in a disproportionate number of patients with advanced disease. DBS is an effective therapy in this population and will become more important in clinical practice in the future. The aim of this review was to provide an update regarding the current clinical status of DBS in the treatment of PD, as well as its future prospects in Korea.
INTRODUCTION
- In 2000, DBS was approved, and the first procedure in Korea was performed. At that time the total cost of DBS was paid by the patients; the financial burden imposed by the procedure was one of the biggest obstacles limiting the accessibility of this surgical intervention. Additionally, DBS was initially introduced by neurosurgeons, and many neurologists were skeptical regarding both the safety and the efficacy of DBS and were therefore reluctant to refer patients for the procedure. Therefore, the number of the new patients treated annually by DBS was less than 50 during the initial years of its use. National Health Insurance (NHI) of Korea is a quasi-governmental organization and an exclusive provider of health care insurance. In 2005, NHI began reimbursing patients for DBS. DBS has gradually become more accepted by neurosurgeons and neurologists. As a result, the number of operations increased, as more than 150 new patients per year opted for procedure; the cumulative number of patients who received DBS reached approximately 2,000 (Figure 1) [5]. Currently, the number of new patients receiving DBS annually hovers at approximately 150, according to the statistics provided by the Health Insurance Review and Assessment Service of Korea. As in other countries, DBS was approved for the treatment of movement disorders (i.e., tremor, PD, and dystonia) and psychiatric disorders (i.e., obsessive compulsive disorder). Extraordinarily indications such as epilepsy and pain, which were regarded as experimental at that time, were included in the list for reimbursement. Additionally, indications not listed in the guideline were treated at the discretion of the physicians if the patients agreed to pay the total cost of the procedure, without reimbursement from NHI. This guideline was revised in 2015. Tourette syndrome was added to the list of indications, and indications deviating from those of the guideline were prohibited, even if patients were willing to pay the total cost of the procedure.
- According to the results of an informal survey, approximately 20 institutions perform DBS, and the number of operations for new patients varies from less than 10 cases per year at the majority of institutions to more than 20 cases per year at a few institutions (Figure 2).
CURRENT STATUS OF DBS IN KOREA
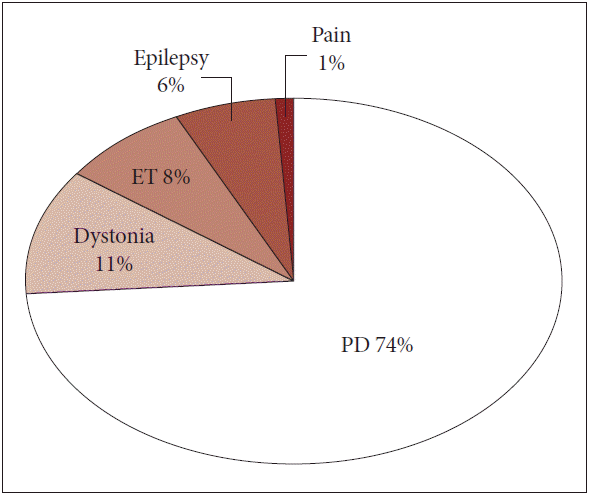
- The most common indication for DBS is PD, which represents 3/4 of cases according to data provided by Medtronic Inc. (Minneapolis, MN, USA) that had been an exclusive supplier of DBS equipment in Korea till (2013). Other indications include dystonia, essential tremor, epilepsy and pain (Figure 3). Because the guidelines for reimbursement by NHI have been strictly enforced nationwide, there is not a wide range of differences in either the indications for DBS or the selection of patients for the procedure. In general, a diagnosis of idiopathic PD with an illness duration longer than 5 years (recently revised to 3 years) and inadequate motor symptom control that responds to levodopa administration are essential components of the guidelines. Although most neurosurgeons prefer to target the subthalamic nucleus (STN), some neurosurgeons have recently advocated targeting the internal globus pallidus (GPi) in selected cases. Simultaneous bilateral surgery is the procedure most often performed and is recommended even in cases of highly asymmetric PD [6]; however, some neurosuregons insist that unilateral DBS may be appropriate for selected patients with asymmetric Parkiknsonism [7]. DBS for intractable tremor-dominant PD was controversial because the original guidelines did not include cases unresponsive to levodopa until recently. Additionally, aside from the STN, GPi, and ventral intermediate nucleus, DBS targeting other areas such as the pedunculopontine nucleus has not been described in Korea.
- The anatomical localization of targets using 1.5-tesla magnetic resonance imaging (MRI) and the physiological identification of targets using microelectrode recording (MER) are the most popular and almost standard surgical methods. The necessity of MER is controversial, and the use of techniques such as single channel MER [8] or simultaneous multichannel [9] or circumferential paired MER [10] vary depending upon the institution at which the procedure is performed or the neurosurgeon who performs the procedure. Advances in imaging technology and surgical devices have allowed for the introduction of new surgical techniques in which MER has been replaced by preoperative and intraoperative imaging. Seven-tesla MRI, which may allow for the improved direct visualization of neural structures, is an example of a recent advance that may be useful in the future [11]. DBS totally depending on imaging remains an unpopular option; however, it is expected that significance of invasive MER will be lessen often with the development of new technology. The method of anesthesia utilized during surgery is another issue of interest. Traditionally, functional stereotactic surgery has been performed in awake patients. DBS has been primarily performed with local anesthesia in awake patients. The rationale for awake surgery is that it maintains the optimal conditions for MER and allows for confirmation of the intended target and adjacent structures via intraoperative stimulation. The withdrawal of anti-Parkinsonian medication before surgery is also commonly used. However, it is difficult to perform surgery in elderly patients and patients with advanced PD who either cannot tolerate the procedure or cannot cooperate with instructions given during surgery. Titrated intravenous anesthesia has become more common for minor surgical procedures, and several new drugs are being used in clinical practice. Intravenous anesthesia using the combination of propofol and fentanyl does not interfere with MER signals from the STN, which improves patients’ experiences [12]. Dexmedetomidine has also been introduced recently.
INDICATIONS, PATIENT SELECTION, AND SURGICAL TECHNIQUES
- Traditionally, DBS programming following surgery has been based on trial and error. Programming guided via fused MRI and CT images is suggested to be a more efficient programming method [13,14]. Routine postoperative programming differs across institutions. Some institutions favor DBS programming immediately following surgery, whereas others prefer to begin programming after a few weeks have passed. The specialization of the caregivers responsible for the programming also varies. In the past, neurosurgeons were responsible for both the surgery and the postoperative programming, the latter of which is extremely time-consuming. As more patients have undergone surgery, neurologists, and nurses specializing in DBS have become increasingly involved in postoperative care, including DBS programming. There is an additional financial issue concerning programming. Currently, NHI reimburses only the initial programming following surgery and does not cover adjustments or reprogramming. The functional benefits of DBS may be maximized when optimal care is guaranteed at each step of DBS, from patient selection to surgery, programming, follow-up and adjustments. A proposal for reimbursement for regular checkups and reprogramming was recently submitted and is awaiting approval.
PROGRAMMING
- The first article regarding DBS for movement disorders written by Korean authors and published in an international peer review journal in 2002 may be retrieved via PubMed [15]. A clinical study article regarding DBS for PD from a Korean institution was first published in 2006 [7,16]. More than 100 papers regarding both clinical and basic research have since been published. Research regarding DBS is increasing, and multiple topics have been explored in the fields of basic and clinical science or medical engineering. Recently published research topics include a quantitative evaluation of Parkinsonian rigidity during DBS [17], the effect of DBS on pain in PD [18], vascular changes after DBS [19], DBS effect on brain network of PD [20], and implantable DBS system [21]. The recent rise of multidisciplinary approaches in both medical and scientific research is expected to foster the development of new devices.
RESEARCH AND ACADEMIC PUBLICATIONS
- In 2005, NHI of Korea began to reimburse DBS; currently, approximately 90% of the cost is reimbursed by NHI, and 10% is paid by the patient. There is an additional fee for the surgeon and the hospital that is not reimbursed by NHI. In the fee for service the reimbursement for medical service are relatively low compared with the cost of the equipment and the material used for the procedure. The total cost of bilateral DBS for PD is approximately 27,500 USD, the majority of which is for coverage of DBS hardware. The minimum cost to the patient may be less than 15% of the total cost (Table 1).
- A recent revision of the guidelines regarding the use of DBS has expanded the number of indications eligible for reimbursement and prohibited the performance of DBS surgery for indications deviating from said guidelines, without exception. Patients must have at least 3 years’ history of disease and respond to levodopa; intractable tremor-dominant PD was recently added to the list of indications. A rapidly aging population and an increasing prevalence of PD may result in an exponentially increased need for DBS in the future. Prolonged life expectancy among affected patients indicates that the number of internal pulse generator (IPG) changes is also likely to increase. Therefore, it is expected that spending for DBS will increase until the aforementioned demographic changes reach a stable equilibrium.
- Until 2013, Medtronic Inc. had been the only supplier of DBS hardware in Korea, before the launch of a competitor (Saint Jude Medical, Plano, TX, USA) in 2014. The reimbursement for hardware is strictly regulated by NHI and tends to be fixed either at the same price or within a narrow range, irrespective of the providing company or the model. Therefore, updated devices with either improved or additional functions such as single IPG supporting both electrodes and rechargeable IPG are not available due to discrepancies between NHI policies intended to reduce financial burdens and the interests of the provider. These obstacles in accessibility to updated technology are inconvenient for patients treated in Korea.
FINANCIAL AND SOCIOECONOMIC ISSUES
- There are several issues that must be addressed further. First, it is evident that DBS is underutilized in Korea when one considers the prevalence of PD and the DBS statistics in other countries. The number of new patients receiving DBS every year in Korea is approximately 150 patients, which is much less than the numbers observed in other countries. For example, the mean number of annual interventions is approximately 80 across 7 centers in Switzerland, which has a total population of approximately 9 million people. Furhermore the authors of the report commented that this is a finding indicative of a conservative practice patternin Switzerland considering prevalence, incidence and the proportion of the patients for which DBS is presumed the best option [22]. US data indicate that 2,500 to 3,000 patients undergo DBS surgery for PD and essential tremor at 200 to 250 centers [23], and additional data suggest that the number may be closer to 4,000 per year [24]. Data from Australia are also indicative of much higher numbers of patients receiving DBS, as between 300 and 350 patients undergo the procedure annually [25]. The reasons for the underutilization of DBS in Korea may be explained by many factors. The cost of DBS remains relatively high, and the financial burden imposed on patients is not trivial, as health insurance does not cover its total cost. Additionally, DBS is not well understood or accepted by either laypeople or neurosurgeons and neurologists. Many neurologists are conservative and are skeptical regarding the use of DBS for their patients. The maintenance of qualified DBS practitioners and the training of competent clinicians and researchers are also important issues. Patient burden is an important factor affecting clinical outcomes. Due to the complexity and precision necessary for DBS for PD, the learning curve is both slow and long. There is no consensus regarding either the minimal or the optimal number of cases that must be performed per year at an individual institution to ensure the quality of the procedure and shorten the surgeon’s learning curve. In a recent study analyzing STN DBS in 233 patients over 14 years, the learning curve for STN DBS for PD lasted two years; complications such as lead misplacement were less frequent following that time period [26]. It has been suggested that a minimum of 200 DBS surgeries is required to be a qualified surgeon and that a case load of 20 per year is considered the minimum number necessary for a training institution [27]. As depicted in Figure 2, the annual case load per institution in Korea is no higher than 10 at many institutions, which appears to be too low to ensure adequate care. Inadequate caseloads also affect resource investments. For example, MER is routinely used in DBS for PD; however, approximately half of the institutions have neither the equipment or dedicated neurophysiologists or technicians for MER. In Switzerland, it was officially decided that DBS would be performed at four centers in 2011 [22]. Although it may be difficult to establish similar regulations or guidelines to qualify institutions for DBS in Korea, open discussions with transparent data collection and assessments are essential to improving care and benefiting larger numbers of patients. Another issue involves the use of a multidisciplinary approach in both clinical medicine and research. The outcomes of DBS for PD are dependent on not only the experience and the skill of the neurosurgeon but also the cooperation of DBS team members from different clinical fields. Needless to say, the neurologist plays an important role in patient selection, pre- and postoperative evaluations, continuation of medical treatment and assessment of patient outcomes. Other members such as psychiatrists, neurophysiologists, rehabilitation doctors, and nursing staffs are also necessary for the performance of DBS. At many institutions, the use of a multidisciplinary approach to improve the quality of care must be more aggressively implemented, as the current level of care provided to patients is not acceptable. A final issue is the enhancement of research activity. There is an increasing need for basic science research and technological improvement as well as clinical research. The economic aspects of DBS are also a concern, given that DBS will likely be used more frequently in the future. There is a huge potential opportunity in this field as an industry when we rememeber the success of Korea in the engineering and the manufacturing sectors. The integration of resources from multiple fields is important in achieving this goal.
DISCUSSION
Figure 1.The annual numbers of new patients who received deep brain stimulation in Korea during the past 5 years according to the statistics of the Health Insurance Review and Assessment Service of Korea [5].
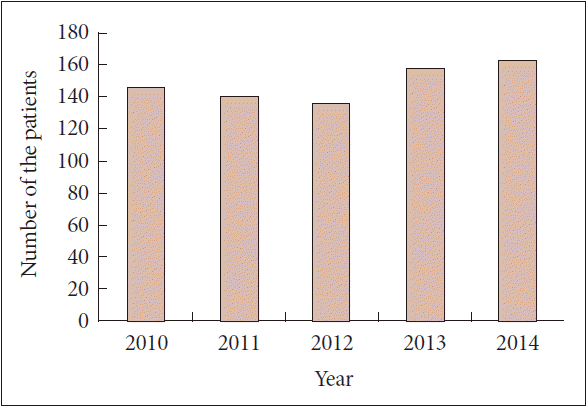
Figure 2.The distribution of the institutions according to the annual numbers of deep brain stimulation procedures for new patients indicates that only 5 of 22 institutions treated more than 10 new patients in 2013.
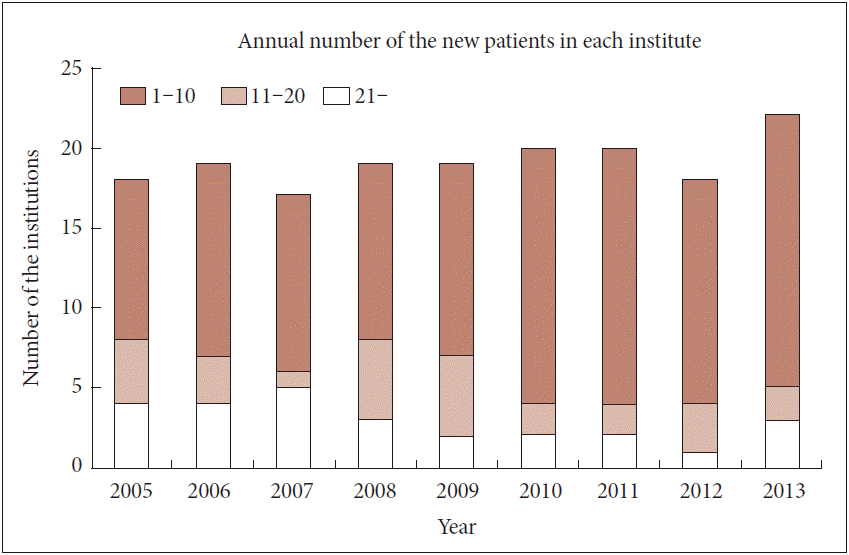
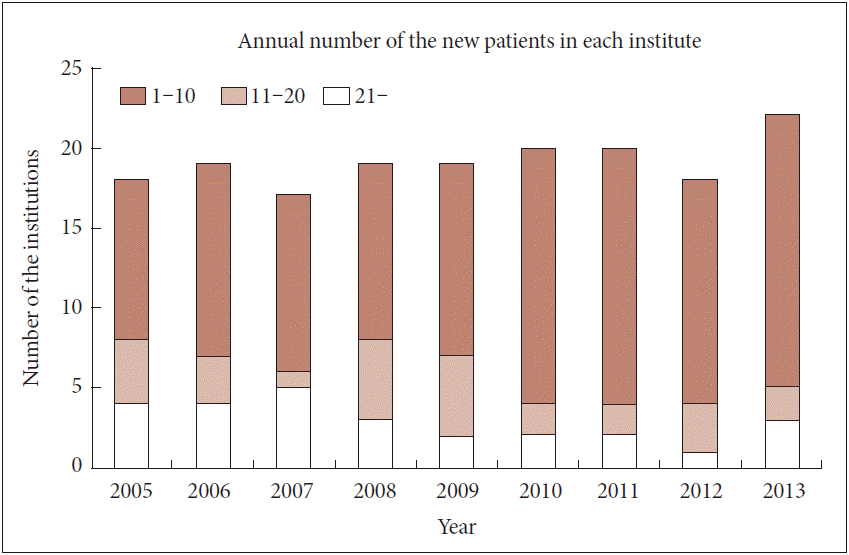
Figure 3.Common indications for deep brain stimulation in Korea (data provided by Medtronic Inc.). ET: essential tremor, PD: Parkinson disease.


Table 1.Direct cost estimates for typical bilateral DBS for PD in a tertiary care hospital
| Cost | |
|---|---|
| Korean Won (KRW)/US Dollars (USD)* | |
| (1) DBS equipment | 2,3443,800/21,313 |
| (2) Surgery | 3,520,450/3,200 |
| (3) Admission cost other than surgery | –2,000,000/–1,818 |
| (4) Other cost not reimbursed by NHI | 1,339,650/1,218 |
| Total | 30,303,900/27,549 |
- 1. Kim JS, Sohn YH. Current status of Parkinson’s disease treatment in Korea. Parkinsonism Relat Disord 2003;9 Suppl 2:S99–S104.Article
- 2. Seo WK, Koh SB, Kim BJ, Yu SW, Park MH, Park KW, et al. Prevalence of Parkinson’s disease in Korea. J Clin Neurosci 2007;14:1155–1157.ArticlePubMed
- 3. Kim JM, Kim JS, Kim KW, Lee SB, Park JH, Lee JJ, et al. Study of the prevalence of Parkinson’s disease using dopamine transporter imaging. Neurol Res 2010;32:845–851.ArticlePubMed
- 4. Statistics Korea. Future population statisitics 2010-2060; Daejeon: Statistics Korea; 2010 48. [cited 2015 Aug 21]. Available from: http://kostat.go.kr/portal/korea/kor_nw/2/2/6/index.board?bmode=read&bSeq=207&aSeq=252623&pageNo=1&rowNum=10&navCount=10&currPg=&sTarget=title&sTxt=.
- 5. Health Insurance Review and Assessment Service. Health-care statistics; Seoul: Health Insurance Review and Assessment Service; 2015 [cited 2015 Aug 21]. Available from: http://www.hira.or.kr/rd/dissdic/infoMdfeeList.do?pgmid=HIRAA020044030000.
- 6. Kim HJ, Paek SH, Kim JY, Lee JY, Lim YH, Kim DG, et al. Two-year follow-up on the effect of unilateral subthalamic deep brain stimulation in highly asymmetric Parkinson’s disease. Mov Disord 2009;24:329–335.ArticlePubMed
- 7. Chung SJ, Jeon SR, Kim SR, Sung YH, Lee MC. Bilateral effects of unilateral subthalamic nucleus deep brain stimulation in advanced Parkinson’s disease. Eur Neurol 2006;56:127–132.ArticlePubMed
- 8. Chang WS, Kim HY, Kim JP, Park YS, Chung SS, Chang JW. Bilateral subthalamic deep brain stimulation using single track microelectrode recording. Acta Neurochir (Wien) 2011;153:1087–1095.ArticlePubMed
- 9. Lee JY, Han JH, Kim HJ, Jeon BS, Kim DG, Paek SH. STN DBS of Advanced Parkinson’s Disease Experienced in a Specialized Monitoring Unit with a Prospective Protocol. J Korean Neurosurg Soc 2008;44:26–35.ArticlePubMedPMC
- 10. Kim MJ, Jeon SR, Kim SR, Lee MC, Chung SJ. Lateralized effects of unilateral thalamotomy and thalamic stimulation in patients with essential tremor. J Mov Disord 2011;4:64–67.ArticlePubMedPMCPDF
- 11. Cho ZH, Min HK, Oh SH, Han JY, Park CW, Chi JG, et al. Direct visualization of deep brain stimulation targets in Parkinson disease with the use of 7-tesla magnetic resonance imaging. J Neurosurg 2010;113:639–647.ArticlePubMedPMC
- 12. Kim W, Song IH, Lim YH, Kim MR, Kim YE, Hwang JH, et al. Influence of propofol and fentanyl on deep brain stimulation of the subthalamic nucleus. J Korean Med Sci 2014;29:1278–1286.ArticlePubMedPMC
- 13. Paek SH, Kim HJ, Yoon JY, Heo JH, Kim C, Kim MR, et al. Fusion image-based programming after subthalamic nucleus deep brain stimulation. World Neurosurg 2011;75:517–524.ArticlePubMed
- 14. Lee JY, Jeon BS, Paek SH, Lim YH, Kim MR, Kim C. Reprogramming guided by the fused images of MRI and CT in subthalamic nucleus stimulation in Parkinson disease. Clin Neurol Neurosurg 2010;112:47–53.ArticlePubMed
- 15. Chang JW, Choi JY, Lee BW, Kang UJ, Chung SS. Unilateral globus pallidus internus stimulation improves delayed onset post-traumatic cervical dystonia with an ipsilateral focal basal ganglia lesion. J Neurol Neurosurg Psychiatry 2002;73:588–590.ArticlePubMedPMC
- 16. Kim MS, Jung YT, Sim JH, Kim SJ, Kim JW, Burchiel KJ. Microelectrode recording: lead point in STN-DBS surgery. Acta Neurochir Suppl 2006;99:37–42.ArticlePubMed
- 17. Kwon Y, Park SH, Kim JW, Ho Y, Jeon HM, Bang MJ, et al. Quantitative evaluation of parkinsonian rigidity during intra-operative deep brain stimulation. Biomed Mater Eng 2014;24:2273–2281.ArticlePubMed
- 18. Jung YJ, Kim HJ, Jeon BS, Park H, Lee WW, Paek SH. An 8-Year Follow-up on the Effect of Subthalamic Nucleus Deep Brain Stimulation on Pain in Parkinson Disease. JAMA Neurol 2015;72:504–510.ArticlePubMed
- 19. Choi BS, Kim YH, Jeon SR. Vascular changes caused by deep brain stimulation using double-dose gadolinium-enhanced brain MRI. Neural Regen Res 2014;9:276–279.ArticlePubMedPMC
- 20. Park HJ, Park B, Kim HY, Oh MK, Kim JI, Yoon M, et al. A network analysis of 15O-H2O PET reveals deep brain stimulation effects on brain network of Parkinson’s disease. Yonsei Med J 2015;56:726–736.ArticlePubMedPMC
- 21. Heo MS, Moon HS, Kim HC, Park HW, Lim YH, Paek SH. Fully Implantable Deep Brain Stimulation System with Wireless Power Transmission for Long-term Use in Rodent Models of Parkinson’s Disease. J Korean Neurosurg Soc 2015;57:152–158.ArticlePubMedPMC
- 22. Christen M, Müller S. Current status and future challenges of deep brain stimulation in Switzerland. Swiss Med Wkly 2012;142:w13570.ArticlePubMed
- 23. Pilitsis JG, Burrows A, Peters ML, Sargent J, Ng SC, Tseng JF. Changing practice patterns of deep brain stimulation in Parkinson’s disease and essential tremor in the USA. Stereotact Funct Neurosurg 2012;90:25–29.ArticlePubMed
- 24. Lad SP, Kalanithi PS, Patil CG, Itthimathin P, Batya S, Bronte-Stewart H, et al. Socioeconomic Trends in Deep Brain Stimulation (DBS) Surgery. Neuromodulation 2010;13:182–186.ArticlePubMed
- 25. Poortvliet PC, Silburn PA, Coyne TJ, Chenery HJ. Deep brain stimulation for Parkinson disease in Australia: current scientific and clinical status. Intern Med J 2015;45:134–139.ArticlePubMed
- 26. Seijo F, Alvarez de Eulate Beramendi S, Santamarta Liébana E, Lozano Aragoneses B, Saiz Ayala A, Fernández de León R, et al. Surgical adverse events of deep brain stimulation in the subthalamic nucleus of patients with Parkinson’s disease. The learning curve and the pitfalls. Acta Neurochir (Wien) 2014;156:1505–1512.discussion 1512. ArticlePubMed
- 27. Krauss JK, Broggi B, Reulen HJ, Trojanowski T, Lazorthes Y; ESSN, et al. Training chart in movement disorders surgery added competence: as approved by the ESSFN and UEMS Section of Neurosurgery (March 2009). Acta Neurochir (Wien) 2009;151:1505–1509.ArticlePubMed
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Immunomodulatory and endocrine effects of deep brain stimulation and spinal cord stimulation – A systematic review
Oskar Puk, Magdalena Jabłońska, Paweł Sokal
Biomedicine & Pharmacotherapy.2023; 168: 115732. CrossRef - Practice Trends of Neuromodulation Therapies for Pain and Spasticity in India
Preeti P. Doshi, Marc Russo, Paresh K. Doshi
Neuromodulation: Technology at the Neural Interface.2021;[Epub] CrossRef - An International Survey of Deep Brain Stimulation Utilization in Asia and Oceania: The DBS Think Tank East
Chencheng Zhang, Adolfo Ramirez-Zamora, Fangang Meng, Zhengyu Lin, Yijie Lai, Dianyou Li, Jinwoo Chang, Takashi Morishita, Tooru Inoue, Shinsuke Fujioka, Genko Oyama, Terry Coyne, Valerie Voon, Paresh K. Doshi, Yiwen Wu, Jun Liu, Bhavana Patel, Leonardo A
Frontiers in Human Neuroscience.2020;[Epub] CrossRef - Canadian Assessment of Deep Brain Stimulation Access: The Canada Study
C. Michael Honey, Armaan K. Malhotra, Mandeep S. Tamber, Michel Prud’homme, Ivar Mendez, Christopher R. Honey
Canadian Journal of Neurological Sciences / Journal Canadien des Sciences Neurologiques.2018; 45(5): 553. CrossRef - Association of Parkinsonism or Parkinson Disease with Polypharmacy in the Year Preceding Diagnosis: A Nested Case–Control Study in South Korea
Hae-Young Park, Ji-Won Park, Hyun Soon Sohn, Jin-Won Kwon
Drug Safety.2017; 40(11): 1109. CrossRef - La Enfermedad de Parkinson: Etiología, Tratamientos y Factores Preventivos
F Hurtado, Melissa Andrea N Cardenas, Fernando Cardenas, Laura Andrea León
Universitas Psychologica.2017;[Epub] CrossRef - Isolated dystypia after subthalamic nucleus deep brain stimulation in a patient with Parkinson’s disease
Si-Hoon Lee, Kipyung Jeon, Byung-chul Son, Joong-Seok Kim
Acta Neurochirurgica.2016; 158(4): 783. CrossRef
Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite