Articles
- Page Path
- HOME > J Mov Disord > Volume 11(1); 2018 > Article
-
Original Article
Quantitative Assessment of Hand Dysfunction in Patients with Early Parkinson’s Disease and Focal Hand Dystonia - Deepa Kandaswamy1, MuthuKumar M1, Mathew Alexander2, Krishna Prabhu3, Mahasampath Gowri S4, Srinivasa Babu Krothapalli1
-
Journal of Movement Disorders 2018;11(1):35-44.
DOI: https://doi.org/10.14802/jmd.17046
Published online: January 11, 2018
1Neurophysiology Laboratory, Department of Neurological Sciences, Christian Medical College, Vellore, India
2Neurology Division, Department of Neurological Sciences, Christian Medical College, Vellore, India
3Neurosurgery Division, Department of Neurological Sciences, Christian Medical College, Vellore, India
4Department of Biostatistics, Christian Medical College, Vellore, India
- Corresponding author: Srinivasa Babu Krothapalli, Neurophysiology Laboratory, Department of Neurological Sciences, Christian Medical College, Vellore-632004, India / Tel: +91 416 2282442 / Fax: +91 416 2232035 / E-mail: srinivas@cmcvellore.ac.in
Copyright © 2018 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objective
- Motor impairments related to hand function are common symptoms in patients with movement disorders, such as Parkinson’s disease (PD) and focal hand dystonia (FHD). However, hand dysfunction has not been quantitatively assessed as a clinical tool for screening patient groups from healthy controls (HCs). The aim of our study was 1) to quantitatively assess hand dysfunction in patients with PD and FHD and its usefulness as a screening tool 2) to grade disease severity in PD and FHD based on hand dysfunction.
-
Methods
- The current case-control study included HCs (n = 50) and patients with known history of PD (n = 25) or FHD (n = 16). Hand function was assessed by a precision grip task while participants lifted objects of 1.3 N and 1.7 N under dry skin conditions, followed by very wet skin conditions (VWSCs). Receiver operating characteristic and summative scoring analyses were performed.
-
Results
- In PD, the combination of loading phase duration and lifting phase duration at quantitative cutoffs of 0.36 and 0.74 seconds identified 21/25 patients as diseased and 49/50 subjects as HCs with 1.7 N under VWSCs. In PD, 5/21 was graded as “mild” and 16/21 as “moderate cases.” In FHD, slip force at a cutoff of 1.2 N identified 13/16 patients as diseased and 41/50 subjects as HC with 1.7 N under VWSCs, but disease severity could not be graded.
-
Conclusion
- Our results demonstrate the use of precision grip task as an important clinical tool in assessment of hand dysfunction in movement disorder patients. Use of quantitative cutoffs may improve diagnostic accuracy and serve as a valuable adjunct to existing clinical assessment methods.
- Subjects
- The current study included male subjects with PD (n = 25, mean age 54.4 ± 8.9 years, range 37–65 years) and age –matched with HCs (n = 50, mean age = 51.8 ± 8.4, age range 36–64 years). Additionally, male participants with FHD (n = 16, mean age 43.9 ± 13.2, age range 25–64 years) and age-matched HCs (n = 50, mean age = 49.4 ± 9.6, age range 26–64 years) participated in the study. The control group was selected if the participants were aged between 20–65 years, had no history of neurological disorders, nerve injuries or hand dysfunction. Cases were selected based on previous clinical diagnosis of PD and FHD (writer’s cramp) by their treating physician based on medical history and neurological examination. All patients with PD were examined in the ‘On’ medication condition. FHD patients treated with botulinum toxin injections > 6 months after the last injection, participated in the current study. Patients were included in the PD category, if they were clinically evaluated to be below stage 2.5, according to Modified Hoehn and Yahr staging [17]. Patients were included under the FHD category if they were clinically evaluated to be below stage 3, according to Fahn-Marsden scaling [18,22,23]. The study was reviewed and approved by the Institutional Review Board (IRB No. 7460) and Institutional Ethics Committee of Christian Medical College, Vellore, India. All participants provided written informed consent before participation.
- Experimental setup and procedure
- Subjects were asked to lift a rectangular object that was placed on a table and limited by an outer steel frame, as shown in Figure 1. The contact surfaces of the object were covered with smooth glass. The object weight could be changed by inserting an exchangeable mass on top of the customized object (7 × 5 × 3 cm). A touch pad with a microswitch underneath it was placed in front of the object. The microswitch was connected to a light-emitting diode that glowed when a hand rested on the touch pad, which marked the beginning and end of a trial. Grip force applied to the object was measured using a miniature load cell (BG-1000G; Kulite, Leonia, NJ, USA) placed inside the object. A 3 axis (± 3 g range) accelerometer (ADXL335; Analog Devices, Norwood, MA, USA) embedded in the object measured object acceleration. A rubber band wound around the steel frame indicated the height to which the subject was required to lift the object. Position of the object was monitored by an infrared distance-sensing module (GP2D120; Sharp Microelectronics, Munich, Germany) that was hung from the top of the steel frame.
- All participants were instructed to wash their hands and naturally dry them before commencing the proposed task. Each individual was allowed practice trials where they were asked to reach, grip the object between the thumb and index finger, lift to a height of 12 cm, hold stationary for 5 seconds and on command by the experimenter allowed it to slip by gradual reduction of grip force. The slip events were identified by vertical object motion shown by the accelerometer signal, with a decline in grip force. Thus, the trials with slips were used for calculating the slip force (SF). An inter-trial interval of 10 seconds was given. All the subjects were tested for two different weights (1.3 N and 1.7 N) under two different skin conditions [dry skin condition (DSC) and very wet skin condition (VWSC)]. Friction between the skin and the grasped object is highly variable due to inter-subject variation in moisture (sweat) content and across trials for the same subject [24-26]. Such variations in the coefficient of skin friction alter the grip force applied during object manipulation in a precision grip task. A pilot study conducted on HCs (n = 80) suggested that the coefficient of variation of friction significantly reduced when subjects performed the precision grip task in VWSC for all the object weights. Based on this rationale, we decided to conduct this study under both DSC and VWSC. Under each condition six trials were performed. For VWSC, subjects were allowed to dip their thumb and index finger in a tray filled with distilled water for 10 seconds before commencement of each trial.
- Patients with movement disorders had difficulty in handling common objects used in daily routine. To mimic the weight of such commonly used objects, we chose objects of lower weights (< 2 N) for the study. Only two object weights (1.3 N and 1.7 N) were considered for the current study (from a series of four weights taken for a pilot study whose data are not shown here).
- A customized user interface was used to record the signals using LabVIEW software 2010 (National Instruments, Austin, TX, USA) and the digitized signals were acquired using a 16-bit ADC (Multi-channel Data Acquisition Card NI PCI 6014; National Instruments) at 1,000 samples per second for each channel. A laboratory computer stored the signal traces of grip force, position of the object, three axis accelerometer data and touch pad events. The force transducer signals were conditioned (i.e., signals were amplified and passed through low pass first order Butterworth filter at cut of frequencies of 16 Hz). Data were extracted and analyzed using MATLAB (MathWorks Inc., Natick, MA, USA) based on a customized program. Acquired raw signals of force, rate and temporal parameters for each trial were extracted using customized algorithms in MATLAB. The lifting task was segmented into particular time windows. T1–T2 was marked as the point of first contact of the object to the beginning of movement of the object from the table (loading phase), T2–T3 as the time from the onset of object’s vertical movement to its intended height, and T4–T5 as time taken to hold the object in hand. Force, temporal and rate parameters were extracted for evaluating hand dysfunction. Force [expressed in Newtons (N)] parameters were peak grip force, stable grip force, grip force at loading phase and SF. Temporal (expressed in milliseconds) parameters included loading phase duration (LPD), time taken to reach peak grip force (TPGF), and lifting phase duration (LFD). Rate of grip force applied on the object (Newton/sec) and rate of lift (meters/sec) are the rate profiles.
- Statistical analysis
- Baseline characteristics of normally distributed variables are summarized as the mean ± standard deviation (SD). Parameters with optimized cutoff values were identified by receiver operating characteristic (ROC) analysis. ROC curves were constructed for force, rate and temporal parameters for both object weights (1.3 N and 1.7 N), and skin conditions (DSC and VWSC) in all subjects. The area under the curve (AUC), sensitivity and specificity were evaluated by ROC analysis. Precision grip parameters, which showed a high percentage of both sensitivity and specificity, were only considered optimal and used for accurate classification of subjects with PD, FHD or HC. Positive predictive value (PPV) and negative predictive value (NPV) with 95% confidence interval (CI), positive and negative likelihood ratios (LR+/LR-) were calculated.
- To grade the severity of the disease, a summative scoring analysis was performed.
- A score of 1 (one) was assigned for the optimized parameters (derived from the ROC analysis) and the assigned scores were summed. Then, the summative score obtained for each patient by precision grip task was compared with the “gold standard clinical grade” (i.e., Modified Hoehn and Yahr staging). Based on this comparison, the disease severity in patients was graded as “mild” or “moderate” cases. All analysis was performed using SPSS Version 16.0 (SPSS Inc., Chicago, IL, USA) and STATA version 13.1/IC (Stata Corp LP, College Station, TX, USA). The level of significance was set to p < 0.05.
MATERIALS & METHODS
- Characteristics of the study participants
- Table 1 presents demographic and clinical characteristics of HCs and PD patients. The means ± SDs of the ages of subjects with PD and their controls were 54.4 ± 8.9 years and 51.8 ± 8.4, respectively. The mean difference in age between the patients with PDs and HC was 2.6 years, which was not statistically significant (p = 0.3). The duration of PD diagnosis ranged between 0.5–3 years with a mean of 2.0 ± 0.7 years, and most scored between 1–2.5, according to Modified Hoehn and Yahr staging. Table 1 shows that among 16 subjects with FHD, the mean ± SD of age was 43.9 ± 13.2 years, and their corresponding controls were aged 49.4 ± 9.6 years. The mean age difference between the patients with FHD and HC was 5.5 years, which was not statistically significant (p = 0.06). The average duration of diagnosis was 3 years and the patients had a Fahn-Marsden scale between 2 and 3.
- Optimal parameters and discriminatory cutoff values for PD and HC subjects (Table 2)
- In the ROC analysis, for all object weights and skin conditions, TPGF, LPD and LFD showed high sensitivity, specificity and AUC; therefore, they were considered “optimal parameters.” Among the three parameters, TPGF consistently demonstrated higher sensitivity, specificity and AUC under all conditions examined, with AUC within a narrow range of 94–95%. At a cutoff of 0.38 seconds, the sensitivity, specificity and AUC for TPGF at 1.3 N DSC was 92, 90, and 94% respectively. At 1.3 N VWSC, the optimal cutoff was slightly lower (0.34 seconds) but yielded optimal sensitivity (84%), specificity (84%), and AUC (95%). Compared with 1.3 N under DSC, TPGF at 1.7 N under DSC showed relatively lower sensitivity, specificity and AUC at a slightly higher cutoff of 0.40 seconds. The maximal sensitivity (88%), specificity (88%), and AUC (94%) for TPGF was observed at 1.7 N VWSC with a maximal cutoff of 0.44 seconds compared with those for all other settings.
- The optimal cutoff for LFD was highest compared with that for TPGF and LPD under both DSC and VWSC and both 1.3 N and 1.7 N. With an increase in object weights from 1.3 N to 1.7 N under both skin conditions, LFD showed increased cutoff values with improved sensitivity, specificity and AUC (Table 2). In contrast, the cutoffs for LPD were consistently lower than those for TPGF and LPD across all experimental conditions, albeit a large AUC ranging from 91–93%. Compared with all parameters, TPGF showed the highest sensitivity (92%), specificity (90%), and AUC (94%) for 1.3 N DSC. However, on a cumulative basis, all three optimal parameters showed relatively higher sensitivity, specificity and AUC during lifting of an object weighing 1.7 N in VWSC.
- Thus, at an optimal weight of 1.7 N and VWSC, time to reach peak grip force, LPD and LFD screened 21, 20, and 22 of 25 patients, respectively; moreover, they correctly identified 45, 43, and 42 of 50 controls at optimal cutoff values of 0.44, 0.36, and 0.74 seconds, respectively (Table 2).
- To enhance the diagnostic accuracy, we retested ROC analysis by combining the optimal parameters (TPGF, LPD, and LFD under 1.7 N VWSC) in different possible combinations with an aim to test if sensitivity, specificity or AUC could further improve with parameter combinations rather than individual parameters. Based on results from Figure 2A and Table 2, compared with each optimal parameter under 1.7 N VWSC, only a combination of LPD (0.36 seconds) and LFD (0.74 seconds) improved specificity to 98% (95% CI: 89.4–99.9) (Table 2). Thus, the detection rate of HCs significantly improved from 45/50 (Table 2) to 49/50. Our results have laid equal emphasis also in correctly screening the maximum number of subjects without disease as controls. Thus, a total of 21/25 patients were also correctly identified as diseased with sensitivity 84% (95% CI: 63.9–95.5), AUC 91%, PPV 95.5% (95% CI: 77.2–99.9), and NPV 92.5% (95% CI: 81.8–97.9) by combining LPD and LFD at 1.7 N VWSC (Table 2).
- On average, the time taken by HCs during LPD was 0.25 seconds and 0.55 seconds during LFD, while PD patients took 0.71 and 1.57 seconds, respectively (Figure 2B). Since the cutoff values for LPD and LFD were 0.36 and 0.74 seconds, respectively, the average time taken by HCs to lift the object seemed to be well within the determined cutoff values, while PD patients took more time, indicating temporal latency.
- Optimal parameters and discriminatory cutoff values for FHD and HC subjects
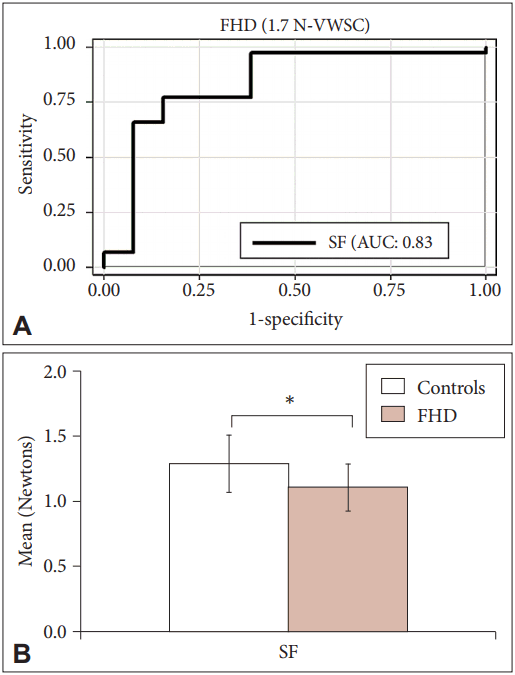
- Unlike PD, in the ROC analysis performed for both object weights and skin conditions in FHD patients, SF and LPD showed high sensitivity, specificity and AUC for both 1.3 N and 1.7 N under VWSC (Table 3). Thus, these were considered the “optimal parameters” for screening FHD. SF showed maximal AUC of 84% for object weight 1.3 N VWSC. The corresponding cutoff was 0.93 N and the sensitivity and specificity were 80%. At this cutoff, 11/16 cases and 33/50 controls were classified appropriately. The LPD for the same setup showed a comparatively lower AUC (80%) at similar sensitivity and specificity and identified 40/50 controls. At comparable sensitivity and specificity, both SF and LPD at object weight 1.7 N and VWSC displayed AUCs of 83% and 74%, respectively. Comparing the sensitivity, specificity and AUC of the optimal parameters between 1.3 N and 1.7 N VWSC, SF independently showed improved sensitivity of 84% with 79% specificity and 83% AUC for 1.7 N VWSC (Figure 3A). Overall, SF showed maximal sensitivity with a cutoff of 1.2 N for 1.7 N VWSC, and therefore this was chosen as an individual optimal parameter for screening FHD from HCs. In this context, it is important to note that a maximum of 13/16 were screened as diseased and 41/50 as HCs (Table 3).
- However, as in the case of PD, the optimal parameters (SF and LPD) were combined, and ROC analysis was tested to ensure if the diagnostic accuracy of FHD could be further improved (data not shown). The percentage of sensitivity, specificity and AUC did not significantly improve.
- Based on Figure 3B, the SFs applied on an average by HCs and FHD patients were 1.3 N and 1.1 N, respectively. The SF of HCs was above the identified cutoff values (1.2 N), while it was below for the FHD patients at 1.7 N VWSC, showing that the SFs applied by FHD patients were less than those applied by HCs.
- Grading disease severity
- The temporal parameters used for screening the maximum number of PD patients were taken into consideration for grading disease severity. According to the summative scoring analysis, a score of 1 was assigned to any individual who had either a LPD value above 0.36 seconds or LFD value above 0.74 seconds, respectively. Subjects with both values above the given cutoff assume a summative score of 2 and a score of zero if either LPD or LFD does not exceed the cutoff. Based on such score assignments, we observed that 5/22 PD patients with a summative score of 1 corresponded to 1 or 1.5 of Hoehn and Yahr grading. Similarly, 16/22 PD patients with a summative score of 2 corresponded to 2 on Hoehn and Yahr staging, allowing grading of 5 patients as “mild” and 16 patients as “moderate.”
- The SF used for screening the maximum number of dystonia patients as diseased was considered for grading disease severity. Based on summative scoring analysis, a score of 1 was assigned to the single optimal parameter, the SF. Comparing the summative scores obtained by precision grip task with the clinical Marsden scaling of dystonia patients, the results were not statistically significant enough to classify them as “mild” and “moderate” cases. Therefore, the severity of the disease could not be graded among FHD patients.
- Among the 25 patients with PD, 7 were clinically graded (Modified Hoehn and Yahr staging) with a score of 1 or 1.5, and 18 with a score of 2. However, by precision grip task scoring two patients from each score category could not be graded. Therefore, 5/7 and 16/18 could be graded by precision grip scoring as “mild” and “moderate” cases, respectively. This implied that 71.4% of patients were graded as “mild” and 88.9% were correctly graded as “moderate” cases. Association between the Modified Hoehn and Yahr clinical grading and precision grip task scoring was found to be statistically significant (p ≤ 0.01). Additionally, the concordance rate between the clinician grading and the precision grip tool was calculated to be 87.5% (i.e., 22/25 were graded). Thus, a significant correlation was found between the precision grip task grading score and the Modified Hoehn and Yahr staging of the disease (gold standard) for PD patients. However, the correlation for FHD was not performed, as the precision grip task could not grade the disease severity in them.
RESULTS
- In the current study, we systematically evaluated precision grip task parameters and derived optimal cutoff values, which may aid in early diagnosis of PD and FHD. In patients with PD and FHD, the optimal parameters that could enhance early diagnosis were identified as temporal latency (LPD and LFD) and SF. To our knowledge, we provide the first report using optimal precision grip task parameters with quantitative cutoff values, both in identifying diseased from controls and grading the severity of disease.
- In patients with PD, ROC analysis performed for both object weights and skin conditions showed sensitivity and specificity of approximately 60–80% for force and rate parameters, while temporal parameters ranged between 80–95%. For an object weight of 1.7 N, under VWSC, combination of the temporal parameters namely, LPD and LFD improved the screening accuracy with a sensitivity of 84%, specificity of 98% and AUC of 91%. The time taken by PD patients for LPD (0.71 seconds) and LFD (1.57 seconds) was greater than that taken by HCs (0.25 seconds and 0.55 seconds) for similar parameter sets. Specifically, the temporal latency signified delayed performance during both reaching and lifting components in a precision grip task by the PD patients. Additionally, the summated scores obtained in a similar task for same parameters correlated well with the clinical gold standards (modified Hoehn and Yahr staging). This clearly indicated that the temporal latency with specific cutoff values might be a good marker for identifying early onset of PD and grading the disease severity.
- In patients with FHD, the ROC analysis for both object weights and skin conditions showed sensitivity and specificity of approximately 40–60% for rate and time parameters, while force parameters ranged between 53–73%. For object weight of 1.7 N VWSC, a single parameter, SF, enhanced diagnostic accuracy with 84% sensitivity, 79% specificity and 83% AUC. The SF required by the FHD patients (1.1 N) to release the object gradually from the hand was less than the cutoff value of 1.2 N, while the HCs applied higher SFs (1.3 N). Application of SF is suggestive of voluntary muscle relaxation of the hand. Motor inhibition or preparatory process of the cortex to voluntarily relax the hand muscles has been evidently demonstrated in FHD [27]. Therefore, application of SF below the cutoff estimate by dystonia patients may be attributed to motor inhibition. However, grading the severity of the disease in FHD patients could not be achieved in this study, perhaps due to a small sample size.
- Temporal latency was also observed when patients with PD were assessed with other varied task complexities, such as clockwise/anticlockwise rotation [6], sinusoidal force tracking [10], maximal voluntary contraction [28], and dual-condition tasks [6]. Specifically, PD patients took longer time duration to lift objects with lighter loads under three experimental conditions, including unpredictable load changes, constant object load and with predictable constant load, along with visual control of hand and object [29]. Thus, slowness of movement is one of the cardinal signs of Parkinson’s and clinically correlates to bradykinesia.
- Although most studies have characterized temporal latency as innate grip force behavior in PD patients, only Neely et al. [28] has attempted to report quantitative cutoff values for PD patients. Our results differ from the previous report in three important factors. First, Neely et al. used a single parameter, the duration of force pulses produced to distinguish PD patients from HCs with a high sensitivity of 86.67%, specificity of 89.19%, and AUC of 95%. However, the number of PD patients (n = 12) that could be correctly screened from HCs (n = 12) using the above ROC values were not mentioned clearly. In our study, sensitivity of 84%, specificity of 98% and AUC of 91% was used to identify a maximum of 21/25 patients and 49/50 controls. We believe that an effective screening tool should ideally identify the maximum number of patients with disease, as well as identify maximum number of subjects without disease. With this rationale, we chose an optimal percentage of sensitivity and specificity that identifies maximum number of controls and patients. Second, the delay in performing a task clinically correlates with bradykinesia, which is evident when additional complexities are added to simple movement tasks, or in combination of tasks. This was also observed while testing slowness of movement in clinical setups. Studies have also used dual tasks to bring out the motor dysfunction similarly [30]. Our results provide an added advantage of not combining two tasks (precision grip and cognitive task), but rather two parameters from a single precision grip task that were combined, which improves the diagnostic characteristics.
- Thus, quantitative estimates using a precision grip task are able to differentiate two different patient categories that present within the spectrum of movement disorders. Although previous reports have shown impaired sensorimotor integration among patients with movement disorders [31], our patient groups were able to regulate the grip forces during a constant weight lifting task [23]. The explicit differences in the grip force behavior of PD and FHD patients may contribute to the uniqueness of performing the task under VWSC.
- Our findings raise an important question as to why VWSC favored better outcome rather than DSC. Certain physiological and physical changes induced by wetness on the skin of the fingers would have impacted the performance of gripping task, which follows a sequence of events. Human skin, when brought in contact with water or any aqueous solutions, allows seepage of water into the skin followed by swelling and expansion of the skin, making it supple. This increases the contact area and increases sensory input from the mechanoreceptors of the skin. Therefore, haptic perception of wetness may potentially contribute to improved resolution of finger force control [32]. Thus, increased moisture level may be an efficient strategy to elicit subtle motor deficits, which often are missed during clinical examinations. Moreover, handling objects in VWSC may also be a “sensory trick” which probably allows the patients to regulate the grip forces at optimal levels.
- In conclusion, we demonstrate that a combination of LPD and LFD in a precision grip task with specified cutoff values identifies temporal latency as a robust marker for screening patients with early onset PD. Additionally, a single parameter, such as SF, could distinguish patients with FHD from HC. Thus, these parameters serve as potential screening tools for early identification of patients with PD and FHD, as well as aid in early institution of treatment and rehabilitation.
- Thus, our study provides evidence indicating that the precision grip paradigm [29] can be used as a quantitative tool in distinguishing patient groups with hand dysfunction from healthy subjects.
DISCUSSION
- The authors thank the controls and patients for participating in this experiment.
- This research was supported by the Department of Science and Technology (DST), Govt. of India (Ref. No. SR/SO/HS16/2003) and Department of Biotechnology, Govt. of India (DO. No.BT/PR11632/MED/30/162/2008).
Acknowledgments


| Skin condition | Object weight | Parameter (s) | Cutoff | Sensitivity | Specificity | AUC | No. of PD patients screened (out of 25) | No. of controls screened (out of 50) |
|---|---|---|---|---|---|---|---|---|
| Parameters with high sensitivity, specificity and AUC | ||||||||
| DSC | 1.3 N | TPGF | 0.38 | 0.92 | 0.90 | 0.94 | 22 | 45 |
| LPD | 0.31 | 0.84 | 0.84 | 0.93 | 22 | 42 | ||
| LFD | 0.64 | 0.80 | 0.80 | 0.89 | 20 | 40 | ||
| VWSC | 1.3 N | TPGF | 0.34 | 0.84 | 0.84 | 0.95 | 20 | 43 |
| LPD | 0.29 | 0.84 | 0.84 | 0.92 | 21 | 43 | ||
| LFD | 0.64 | 0.80 | 0.80 | 0.86 | 20 | 41 | ||
| DSC | 1.7 N | TPGF | 0.40 | 0.84 | 0.84 | 0.95 | 21 | 42 |
| LPD | 0.31 | 0.84 | 0.84 | 0.93 | 21 | 42 | ||
| LFD | 0.69 | 0.84 | 0.84 | 0.91 | 21 | 44 | ||
| VWSC* | 1.7 N* | TPGF | 0.44 | 0.88* | 0.88* | 0.94* | 21 | 45 |
| LPD | 0.36 | 0.84* | 0.84* | 0.91* | 20 | 43 | ||
| LFD | 0.74 | 0.88* | 0.88* | 0.94* | 22 | 42 | ||
| Combination of temporal parameters distinguished maximum number of PD patients from HCs at 1.7 N VWSC | ||||||||
| VWSC† | 1.7 N† | LPD | 0.36 | 0.84 | 0.98 | 0.91 | 21 | 49 |
| LFD | 0.74 |
* object weight and skin condition which showed high sensitivity, specificity and area under curve,
† object weight and skin condition under which the maximum number of patients with PD can be distinguished from HCs by combining loading and lifting phase duration parameters.
DSC: dry skin condition, VWSC: very wet skin condition, TPGF: time to reach grip force, LPD: loading phase duration, LFD: lifting phase duration, N: Newtons, s: seconds, AUC: area under curve, PD: Parkinson’s disease, HCs: healthy controls.
| Skin condition | Object weight | Parameters | Cutoff | Sensitivity | Specificity | AUC | No. of FHD patients screened (out of 16) | No. of controls screened (out of 50) |
|---|---|---|---|---|---|---|---|---|
| Parameters with high sensitivity, specificity and AUC | ||||||||
| DSC | 1.3 N | SF (N) | 0.68 | 0.68 | 0.67 | 0.69 | 9 | 34 |
| LPD (s) | 0.24 | 0.80 | 0.76 | 0.81 | 12 | 38 | ||
| VWSC* | 1.3 N* | SF (N) | 0.93 | 0.80* | 0.80* | 0.84* | 11 | 33 |
| LPD (s) | 0.27 | 0.80* | 0.80* | 0.80* | 12 | 40 | ||
| DSC | 1.7 N | SF (N) | 0.98 | 0.48 | 0.47 | 0.56 | 7 | 26 |
| LPD (s) | 0.28 | 0.73 | 0.68 | 0.78 | 10 | 34 | ||
| VWSC* | 1.7 N* | SF (N) | 1.2 | 0.84* | 0.79* | 0.83* | 13 | 41 |
| LPD (s) | 0.26 | 0.67* | 0.62* | 0.74* | 9 | 31 | ||
| Slip force distinguished maximum number of FHD patients from HCs at 1.7 N VWSC | ||||||||
| VWSC† | 1.7 N† | SF (N) | 1.2 | 84.6 | 79.5 | 0.83 | 13 | 41 |
* object weights and skin condition which showed high sensitivity, specificity and AUC,
† object weight and skin condition under which the maximum number of patients with FHD were distinguished from HCs.
DSC: dry skin condition, VWSC: very wet skin condition, SF: slip force, LPD: loading phase duration, N: Newtons, s: seconds, AUC: area under curve, FHD: focal hand dystonia, HCs: healthy controls.
- 1. Agostino R, Currà A, Giovannelli M, Modugno N, Manfredi M, Berardelli A. Impairment of individual finger movements in Parkinson’s disease. Mov Disord 2003;18:560–565.ArticlePubMed
- 2. Forssberg H, Ingvarsson PE, Iwasaki N, Johansson RS, Gordon AM. Action tremor during object manipulation in Parkinson’s disease. Mov Disord 2000;15:244–254.ArticlePubMed
- 3. Uitti RJ, Baba Y, Wszolek ZK, Putzke DJ. Defining the Parkinson’s disease phenotype: initial symptoms and baseline characteristics in a clinical cohort. Parkinsonism Relat Disord 2005;11:139–145.ArticlePubMed
- 4. Torres-Russotto D, Perlmutter JS. Focal dystonias of the hand and upper extremity. J Hand Surg Am 2008;33:1657–1658.ArticlePubMedPMC
- 5. Bleton JP, Teremetz M, Vidailhet M, Mesure S, Maier MA, Lindberg PG. Impaired force control in writer’s cramp showing a bilateral deficit in sensorimotor integration. Mov Disord 2014;29:130–134.ArticlePubMed
- 6. Pradhan S, Scherer R, Matsuoka Y, Kelly VE. Grip force modulation characteristics as a marker for clinical disease progression in individuals with Parkinson disease: case-control study. Phys Ther 2015;95:369–379.ArticlePubMedPDF
- 7. Whishaw IQ, Suchowersky O, Davis L, Sarna J, Metz GA, Pellis SM. Impairment of pronation, supination, and body co-ordination in reach-to-grasp tasks in human Parkinson’s disease (PD) reveals homology to deficits in animal models. Behav Brain Res 2002;133:165–176.ArticlePubMed
- 8. Flanagan JR, Johansson RS. Hand movements. In: Ramachandran VS, editor. Encyclopedia of the Human Brain. Vol 2. San Diego: Academic Press; 2002:399–414.
- 9. Johansson RS. Sensory control of dexterous manipulation in humans. In: Wing AM, Haggard P, Flanagan JR, editors. Hand and Brain: The Neurophysiology and Psychology of Hand Movements. San Diego: Academic Press; 1996:381–414.
- 10. Pradhan SD, Brewer BR, Carvell GE, Sparto PJ, Delitto A, Matsuoka Y. Assessment of fine motor control in individuals with Parkinson’s disease using force tracking with a secondary cognitive task. J Neurol Phys Ther 2010;34:32–40.ArticlePubMed
- 11. Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat Rev Neurosci 2008;9:222–234.ArticlePubMedPDF
- 12. Nowak DA, Hermsdörfer J. Grip force behavior during object manipulation in neurological disorders: toward an objective evaluation of manual performance deficits. Mov Disord 2005;20:11–25.ArticlePubMed
- 13. Nowak DA, Hermsdörfer J. Objective evaluation of manual performance deficits in neurological movement disorders. Brain Res Rev 2006;51:108–124.ArticlePubMed
- 14. Nowak DA, Hermsdörfer J, Marquardt C, Fuchs HH. Grip and load force coupling during discrete vertical arm movements with a grasped object in cerebellar atrophy. Exp Brain Res 2002;145:28–39.ArticlePubMed
- 15. Wenzelburger R, Kopper F, Zhang BR, Witt K, Hamel W, Weinert D, et al. Subthalamic nucleus stimulation for Parkinson’s disease preferentially improves akinesia of proximal arm movements compared to finger movements. Mov Disord 2003;18:1162–1169.ArticlePubMed
- 16. Serrien DJ, Burgunder JM, Wiesendanger M. Disturbed sensorimotor processing during control of precision grip in patients with writer’s cramp. Mov Disord 2000;15:965–972.ArticlePubMed
- 17. Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord 2004;19:1020–1028.ArticlePubMed
- 18. Comella CL, Leurgans S, Wuu J, Stebbins GT, Chmura T; Dystonia Study Group. Rating scales for dystonia: a multicenter assessment. Mov Disord 2003;18:303–312.ArticlePubMed
- 19. Wissel J, Kabus C, Wenzel R, Klepsch S, Schwarz U, Nebe A, et al. Botulinum toxin in writer’s cramp: objective response evaluation in 31 patients. J Neurol Neurosurg Psychiatry 1996;61:172–175.ArticlePubMedPMC
- 20. Marsden CD. Parkinson’s disease. Lancet 1990;335:948–952.ArticlePubMed
- 21. Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron 2003;39:889–909.ArticlePubMed
- 22. Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz C, Friedman J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology 1985;35:73–77.ArticlePubMed
- 23. Nowak DA, Rosenkranz K, Topka H, Rothwell J. Disturbances of grip force behaviour in focal hand dystonia: evidence for a generalised impairment of sensory-motor integration? J Neurol Neurosurg Psychiatry 2005;76:953–959.ArticlePubMedPMC
- 24. Cadoret G, Smith AM. Friction, not texture, dictates grip forces used during object manipulation. J Neurophysiol 1996;75:1963–1969.ArticlePubMed
- 25. Forssberg H, Eliasson AC, Kinoshita H, Westling G, Johansson RS. Development of human precision grip. IV. Tactile adaptation of isometric finger forces to the frictional condition. Exp Brain Res 1995;104:323–330.ArticlePubMedPDF
- 26. André T, Lefèvre P, Thonnard JL. Fingertip moisture is optimally modulated during object manipulation. J Neurophysiol 2010;103:402–408.ArticlePubMed
- 27. Yazawa S, Ikeda A, Kaji R, Terada K, Nagamine T, Toma K, et al. Abnormal cortical processing of voluntary muscle relaxation in patients with focal hand dystonia studied by movement-related potentials. Brain 1999;122(Pt 7):1357–1366.ArticlePubMedPDF
- 28. Neely KA, Planetta PJ, Prodoehl J, Corcos DM, Comella CL, Goetz CG, et al. Force control deficits in individuals with Parkinson’s disease, multiple systems atrophy, and progressive supranuclear palsy. PLoS One 2013;8:e58403. ArticlePubMedPMC
- 29. Fellows SJ, Noth J, Schwarz M. Precision grip and Parkinson’s disease. Brain 1998;121(Pt 9):1771–1784.ArticlePubMedPDF
- 30. Ling H, Massey LA, Lees AJ, Brown P, Day BL. Hypokinesia without decrement distinguishes progressive supranuclear palsy from Parkinson’s disease. Brain 2012;135(Pt 4):1141–1153.ArticlePubMedPMCPDF
- 31. Abbruzzese G, Berardelli A. Sensorimotor integration in movement disorders. Mov Disord 2003;18:231–240.ArticlePubMed
- 32. Kareklas K, Nettle D, Smulders TV. Water-induced finger wrinkles improve handling of wet objects. Biol Lett 2013;9:20120999.ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

- Short term effects of contralateral tendon vibration on motor unit discharge rate variability and force steadiness in people with Parkinson’s disease
Changki Kim, Daryl J. Wile, Sarah N. Kraeutner, Kaylee A. Larocque, Jennifer M. Jakobi
Frontiers in Aging Neuroscience.2024;[Epub] CrossRef - COMPARISON OF TRUNK CONTROL, MANUAL DEXTERITY, AND REACTION TIME ACCORDING TO DIFFERENT STATUS OF BALANCE IN PEOPLE WITH PARKINSON DISEASE
Hatice YAKUT, Zülal BEKAR, Tuba MADEN, Süleyman KUTLUHAN
SDÜ Tıp Fakültesi Dergisi.2023; 30(3): 380. CrossRef - Supination/pronation movement quantification using stereoscopic vision based system towards Parkinson’s Disease assessment – A pilot study
Pedro G. Vaz, Ana L. Reis, João Cardoso
Biomedical Signal Processing and Control.2020; 60: 101976. CrossRef - Wuqinxi Exercise Improves Hand Dexterity in Patients with Parkinson’s Disease
Tian Wang, Guiping Xiao, Zhenlan Li, Kuncheng Jie, Mengyue Shen, Yan Jiang, Zhen Wang, Xiangrong Shi, Jie Zhuang, Jiao Liu
Evidence-Based Complementary and Alternative Medicine.2020; 2020: 1. CrossRef - Handling objects with very wet skin reduce variability during precision grip task
Deepa Kandaswamy, Muthukumar Murthy, Mahasampath Gowri S, Mathew Alexander, Srinivasa Babu Krothapalli
Neuroscience Letters.2019; 703: 177. CrossRef - Parkinsonian patients do not utilize probabilistic advance information in a grip-lift task
Leif Trampenau, Johann P. Kuhtz-Buschbeck, Thilo van Eimeren
Parkinsonism & Related Disorders.2019; 65: 67. CrossRef - Sensorimotor Control in Dystonia
Desrochers, Brunfeldt, Sidiropoulos, Kagerer
Brain Sciences.2019; 9(4): 79. CrossRef
Comments on this article
- Figure
- Related articles
-
- Absence of Alpha-Synuclein Aggregation in Patients With Parkinson’s Disease Complicated by Sigmoid Volvulus
- Association Between Gait and Dysautonomia in Patients With De Novo Parkinson’s Disease: Forward Gait Versus Backward Gait
- Umami and Other Taste Perceptions in Patients With Parkinson’s Disease
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite



