Articles
- Page Path
- HOME > J Mov Disord > Volume 6(2); 2013 > Article
-
Case Report
Amantadine Induced Corneal Edema in a Patient with Primary Progressive Freezing of Gait - Young Eun Kima,b, Ji Young Yunb,c, Hui-Jun Yangb,d, Han-Joon Kimb,e, Mee Kum Kimf, Won Ryang Weef, Beom S. Jeonb,e
-
Journal of Movement Disorders 2013;6(2):34-36.
DOI: https://doi.org/10.14802/jmd.13008
Published online: October 30, 2013
aDepartment of Neurology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
bParkinson’s Disease Study Group, Seoul National University Hospital, Seoul, Korea
cDepartment of Neurology, Ewha Womans University Mokdong Hospital, Seoul, Korea
dDepartment of Neurology, Ulsan University Hospital, Ulsan, Korea
eDepartment of Neurology and Movement Disorder Center, Seoul National University College of Medicine, Seoul, Korea
fDepartment of Ophthalmology, Seoul National University College of Medicine, Seoul, Korea
- Corresponding author Beom S. Jeon, MD, PhD, Department of Neurology, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul 110-744, Korea, Tel +82-2-2072-2876, Fax +82-2-3672-7553, E-mail brain@snu.ac.kr
- The authors have no financial conflicts of interest.
Copyright © 2013 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 18,310 Views
- 74 Download
- 7 Crossref
ABSTRACT
- Amantadine is commonly used for Parkinsonism. However amantadine can induce adverse corneal reaction. Here we report a patient with primary progressive freezing of gait who had severe corneal edema associated with amantadine, which was reversible after discontinuation of the amantadine. This report alerts neurologists for this reversible but potentially critical corneal edema in patients with Parkinsonism who are receiving amantadine.
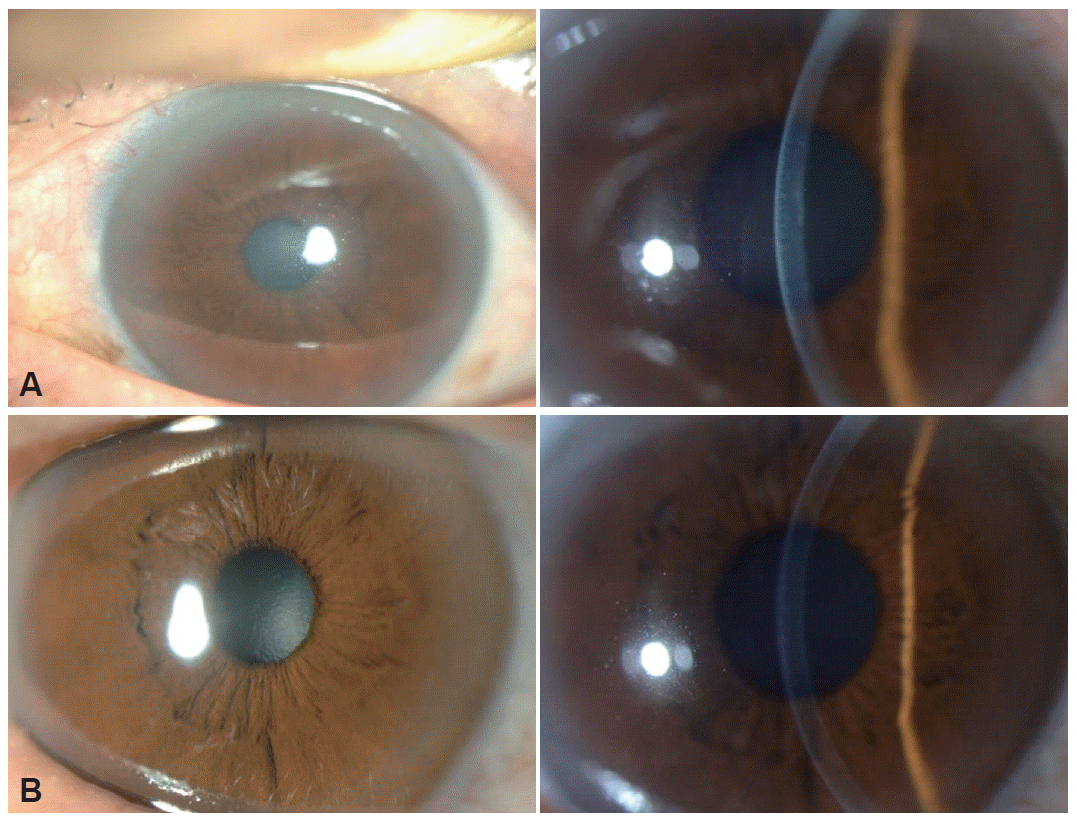
- A 63-year-old woman presented with progressive FOG for 3 years. She had no bradykinesia, rigidity, and tremor, even though she had hypophonia and postural instability. She did not have abnormal ocular movement and autonomic dysfunction. Her cognition andbrain MRI was normal. Her FOG did not respond to levodopa. However, amantadine 400 mg per day was somewhat effective for treating her FOG. After taking amantadine for about 7 months, her bilateral visual acuity had decreased for over 1 week. Accordingly, she visited an eye clinic at another hospital. Severe corneal edema was found in both eyes. Her treating physician recommended corneal transplantation. This information was given to us during her regular follow-up. She was immediately referred to our ophthalmology department. Upon slit lamp examination, profound corneal edema was found (Figure 1A). Corneal evaluation revealed Descemet’s folds, and punctate epithelial erosion as well as profound corneal edema. Her uncorrected visual acuity was 0.15 in the right eye and 0.2 in the left eye. Because amantadine was considered to be the cause of this problem, amantadine was discontinued and the scheduled corneal transplantation was postponed. One month after ceasing the amantadine, her uncorrected visual acuity recovered back to 0.3 in the right eye and 0.7 in the left eye. Best corrected visual acuity was 0.9 and 1.0 in each eye. Her corneal pachymetry (normal value, 0.53–0.55 mm) had improved from 0.661 mm to 0.532 mm in the right eye, and from 0.651 mm to 0.523 mm in the left eye. Although both corneas showed no edema, endothelial cell density (normal density in age range of 60s is mean 2613/mm2)8 was irreversibly decreased (608/mm2 in the right eye and 621/mm2 in the left eye) (Figure 1B).
Case
- There have been few reports on corneal edema associated with amantadine use. All patients experienced bilateral diffuse corneal edema while receiving systemic amantadine therapy at a dose of 100–400 mg a day for a duration ranging from several days to 8 years.6,7,9–18 Corneal edema in most of the cases resolved within 8 days to 2 months after the discontinuation of amantadine.6,7,9–18 However, in our case, endothelial cell density decreased irreversibly after having taken amantadine for 7 months, although the corneal edema improved. Previous case reports identified permanent endothelial cell loss in amantadine users with a long duration from 1 year to 6 years.7,13,14 In addition, a cross-sectional study demonstrated that amantadine users are more likely to have a lower endothelial cell density when used over the long-term.5
- Although the mechanism of the adverse effect of amantadine is not clear, the drug in the tear film, which is secreted form the lacrimal glands, can create superficial corneal deposit, associated epithelial edema and keratitis. In addition, drug hypersensitivity to amantadine and toxic effect to corneal endothelial cells are suggested mechanisms as well.5
- Ophthalmologic adverse events of amantadine are rare and hence, are sometimes underrecognized.10 It is important to detect amantadine associated corneal edema because this side effect is potentially reversible. In addition, regular monitoring of ophthalmology should be recommended to patients receiving amantadine to detect acute and chronic complications. This report alerts neurologists for this reversible but potentially critical corneal edema in patients with Parkinsonism who are receiving amantadine.
Discussion
- This study was supported by a grant from the Korea Health technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A101273).
Acknowledgments

- 1. Gottwald MD, Aminoff MJ. Therapies for dopaminergic-induced dyskinesias in Parkinson disease. Ann Neurol 2011;69:919–927.ArticlePubMed
- 2. Kim YE, Yun JY, Jeon BS. Effect of intravenous amantadine on dopaminergic-drug-resistant freezing of gait. Parkinsonism Relat Disord 2011;17:491–492.ArticlePubMed
- 3. Kim YE, Yun JY, Yang HJ, Kim HJ, Gu N, Yoon SH, et al. Intravenous amantadine for freezing of gait resistant to dopaminergic therapy: a randomized, double-blind, placebo-controlled, cross-over clinical trial. PLoS One 2012;7:e48890.ArticlePubMedPMC
- 4. Malkani R, Zadikoff C, Melen O, Videnovic A, Borushko E, Simuni T. Amantadine for freezing of gait in patients with Parkinson disease. Clin Neuropharmacol 2012;35:266–268.ArticlePubMedPMC
- 5. Chang KC, Jeong JH, Kim MK, Wee WR, Lee JH, Jeon BS. The effect of amantadine on corneal endothelium in subjects with Parkinson’s disease. Ophthalmology 2010;117:1214–1219.ArticlePubMed
- 6. Kubo S, Iwatake A, Ebihara N, Murakami A, Hattori N. Visual impairment in Parkinson’s disease treated with amantadine: case report and review of the literature. Parkinsonism Relat Disord 2008;14:166–169.ArticlePubMed
- 7. Chang KC, Kim MK, Wee WR, Lee JH. Corneal endothelial dysfunction associated with amantadine toxicity. Cornea 2008;27:1182–1185.ArticlePubMed
- 8. Lee DW, Kim JM, Choi CY, Shin D, Park KH, Cho JG. Age-related changes of ocular parameters in Korean subjects. Clin Ophthalmol 2010;4:725–730.ArticlePubMedPMC
- 9. Hughes B, Feiz V, Flynn SB, Brodsky MC. Reversible amantadine-induced corneal edema in an adolescent. Cornea 2004;23:823–824.ArticlePubMed
- 10. Deogaonkar M, Wilson K, Vitek J. Amantadine induced reversible corneal edema. J Clin Neurosci 2011;18:298–299.ArticlePubMed
- 11. Dubow JS, Rezak M, Berman AA. Reversible corneal edema associated with amantadine use: an unrecognized problem. Mov Disord 2008;23:2096–2097.ArticlePubMed
- 12. Hotehama A, Mimura T, Usui T, Kawashima H, Amano S. Sudden onset of amantadine-induced reversible bilateral corneal edema in an elderly patient: case report and literature review. Jpn J Ophthalmol 2011;55:71–74.ArticlePubMed
- 13. Jeng BH, Galor A, Lee MS, Meisler DM, Hollyfield JG, Schoenfield L, et al. Amantadine-associated corneal edema potentially irreversible even after cessation of the medication. Ophthalmology 2008;115:1540–1544.ArticlePubMed
- 14. Koenig SB, McDermott ML, Simons KB. Nonimmunologic graft failure after Descemet’s Stripping Automated Endothelial Keratoplasty (DSAEK) for presumed amantadine-induced corneal edema. Eye Contact Lens 2009;35:209–211.ArticlePubMed
- 15. Pearlman JT, Kadish AH, Ramseyer JC. Vision loss associated with amantadine hydrochloride use. JAMA 1977;237:1200.Article
- 16. Blanchard DL. Amantadine caused corneal edema. Cornea 1990;9:181.Article
- 17. Fraunfelder FT, Meyer SM. Amantadine and corneal deposits. Am J Ophthalmol 1990;110:96–97.ArticlePubMed
- 18. Nogaki H, Morimatsu M. Superficial punctate keratitis and corneal abrasion due to amantadine hydrochloride. J Neurol 1993;240:388–389.ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Amantadine-induced corneal edema: A case and literature review
Antony Raharja, Wessam Mina, Zahra Ashena
American Journal of Ophthalmology Case Reports.2023; 32: 101881. CrossRef - Experience of diagnosis and managements for patients with primary progressive freezing of gait
Li-Li Zhang, Ya-Jie Zhao, Liang Zhang, Xiao-Ping Wang
Journal of Neurorestoratology.2022; : 100039. CrossRef - Toxicity of amantadine hydrochloride on cultured bovine cornea endothelial cells
Po-Yen Lee, Yu-Hung Lai, Po-Len Liu, Ching-Chih Liu, Chia-Cheng Su, Fang-Yen Chiu, Wei-Chung Cheng, Shiuh-Liang Hsu, Kai-Chun Cheng, Li-Yi Chiu, Tzu-En Kao, Chia-Ching Lin, Yo-Chen Chang, Shu-Chi Wang, Chia-Yang Li
Scientific Reports.2021;[Epub] CrossRef - Efficacy and safety of amantadine for the treatment of l-DOPA-induced dyskinesia
Santiago Perez-Lloret, Olivier Rascol
Journal of Neural Transmission.2018; 125(8): 1237. CrossRef - Ocular and visual disorders in Parkinson's disease: Common but frequently overlooked
Merel S. Ekker, Sabine Janssen, Klaus Seppi, Werner Poewe, Nienke M. de Vries, Thomas Theelen, Jorik Nonnekes, Bastiaan R. Bloem
Parkinsonism & Related Disorders.2017; 40: 1. CrossRef - Parkinson’s disease between internal medicine and neurology
Ilona Csoti, Wolfgang H. Jost, Heinz Reichmann
Journal of Neural Transmission.2016; 123(1): 3. CrossRef - Amantadine Use as a Risk Factor for Corneal Edema: A Nationwide Cohort Study in Taiwan
Po Yen Lee, Hung Pin Tu, Chang Ping Lin, Cheng Hsien Chang, Kai Chun Cheng, Chia Ching Lin, Shiuh Liang Hsu
American Journal of Ophthalmology.2016; 171: 122. CrossRef
Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite