Transient Hypersomnolence Provoked by Metoclopramide in a Patient with Degenerative Parkinsonism
Article information
Dear Editor,
Metoclopramide is widely utilized to control nausea and vomiting. Its antiemetic effect is mediated through central blockade of dopamine D2 receptors at the chemoreceptor trigger zone [1]. This property can also induce adverse effects in the central nervous system, such as acute dystonic reactions, akathisia, parkinsonism, psychiatric disturbances and tardive syndrome, in the general population [1-3]. The elimination half-life in individuals with normal renal function following both intravenous and oral administration of a single dose is 5 to 6 hours [4].
Administration of metoclopramide in patients with Parkinson’s disease (PD) worsens parkinsonian motor symptoms. Rarely, metoclopramide-induced acute encephalopathy has been reported in PD [2,3]. Here, we report a case of metoclopramide-induced altered consciousness in a patient with degenerative parkinsonism.
A 78-year-old woman visited our clinic because of her slowly progressive gait disturbance and frequent falls that began 1 year ago. Her body, in general, became rigid with a tendency to fall if not assisted by someone. The patient did not report any nonmotor symptoms such as dream-enacting behavior, hyposmia, visual hallucinations, and cognitive impairment. She had no family history of movement disorders and did not take any drugs such as neuroleptics, prokinetics or calcium channel blockers.
Neurologic examination revealed asymmetric bradykinesia and rigidity predominantly on the left side. Her gait was slow with marked decreases in step and stride lengths. She could barely maintain balance during a pull test. The postural instability and imbalance during walking could not be attributed to ataxia. Gaze paresis was not observed, however, the “round the houses” sign was found.
The MRI revealed diffuse brain atrophy and periventricular white matter changes with Fazekas grade 2. The ventricular size was measured as 0.29 on the Evans index. Basal ganglia structural lesions were not found (Figure 1A). A midline sagittal section of her MRI depicted a “hummingbird sign” with concave superior margin of the midbrain (Figure 1B). Positron emission tomography using 18F-N-(3-fluoropropyl)-2beta-carbon ethoxy-3beta-(4-iodophenyl) nortropane demonstrated asymmetric reduced dopamine transporter uptake at the posterior putamen and caudate, predominantly on the right side (Figure 1C). The blood tests including thyroid function were unremarkable.
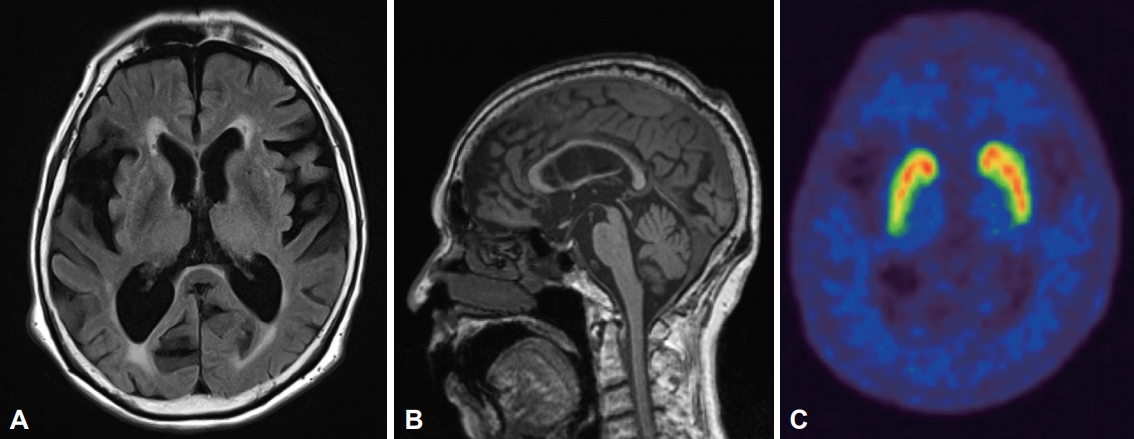
The initial brain MRI revealed Fazekas grade 2 white matter change but without any basal ganglia structural changes. The Evan’s Index was 0.29 (A). Midline sagittal T1-weighted imaging depicts a “Hummingbird” sign with concave superior margin of the midbrain (B). Positron emission tomography using 18F-N-(3-fluoropropyl)-2beta-carbon ethoxy-3beta-(4-iodophenyl) nortropane demonstrated asymmetric reduced dopamine transporter uptake at the posterior putamen and caudate, predominantly on the right side (C).
A tentative diagnosis was made for degenerative parkinsonism due to the suggestive features of progressive supranuclear palsy (PSP) [5]. Levodopa was slowly titrated to a levodopa equivalent daily dose of 1,200 mg; however, the patient reported neither drug adverse effects nor any improvements in her motor symptoms.
While carefully monitoring drug response, she visited the emergency department because of dizziness and nausea. The symptoms were not temporally related to drug intake nor postural change. The patient was fully alert and afebrile upon arrival. A mixture of 10 mg of metoclopramide with 100 mL of saline was continuously given intravenously over a period of 30 minutes by an emergency medicine physician. Immediately after the infusion, the patient quickly developed irresistible and excessive sleepiness, of which she never experienced before, and her mental status altered to a deep drowsy status. Upon neurologic examination, she was able to localize pain when forceful pressure was applied to her sternum. She was unresponsive to verbal stimuli except for vigorous painful stimuli. Her mild arousal was short-lived only during painful stimuli, and she quickly returned to her previous state of sleepiness. There were no abnormal lateralizing and localizing signs found for the organic brain lesions. Blood tests excluded any metabolic or infectious causes. Diffusion-weighted imaging for discrimination of any acute structural abnormalities did not reveal any acute structural lesions to explain her altered mental state.
The patient was hydrated with normal saline solution without any other medications, and her mental status was recovered to an alert state spontaneously after approximately 10 hours.
Encephalopathy is a rare CNS adverse event of metoclopramide, and this case report is peculiar for PD patients [2,3]. Metoclopramide-induced encephalopathy merely suggests that a dopaminergic deficiency in PD rendered the PD patient more sensitive and susceptible to the drug’s dopamine antagonism [2,3]. PSP is a neurodegenerative disease that involves nigrostriatal degeneration; it also encompasses extranigral areas that are responsible for its symptomatology [5]. This implies that PSP lacks dopamine activities at presynaptic and postsynaptic levels. Thus, our patient may be more vulnerable to metoclopramide’s dopaminergic antagonism.
The patient’s decreased consciousness may be explained by extrapolating from the mechanism of modafinil, a drug administered to reverse sleepiness. Modafinil promotes wakefulness by inhibiting the dopamine transporter. Through its inhibition, it elevates synaptic dopamine levels which in turn increases tonic firing and downstream effects on other neurotransmitters such as histamine and hypocretin [6]. The pharmacodynamics of modafinil suggest that the antidopaminergic action of metoclopramide could impair levels of consciousness. Dopamine also shares structural similarities with several wake-promoting drugs, and low-dose dopamine stimulation promotes both slow wave and REM sleep in addition to inducing somnolence [7]. Metoclopramide’s dopaminergic antagonism therefore acts against wake-promoting mechanisms and provides a hypodopaminergic state that could disturb the sleep-wake physiology, all of which strengthens our reasoning in explaining the patient’s phenomenon. The ventral tegmental area receives projections from hypocretin neurons, the pedunculopontine nucleus, and the extended amygdala. This area relays the projections towards thalamocortical arousal via the thalamus and limbic system [7]. The pathologic involvement of the ventral tegmental area, periaqueductal gray and raphe nucleus in PSP further strengthens our reasoning for explaining the patient’s reaction to metoclopramide taken at an extremely low dosage.
We did not exclude an epileptic nonconvulsive seizure through electroencephalography. However, the patient’s prompt response to pain and spontaneous recovery clinically excluded the possibility of epileptic phenomena. We also ruled out the possibility of a “sleep attack” induced by dopamine therapy because the patient skipped the scheduled medication due to dizziness and nausea. The patient’s decreased arousal occurring immediately after metoclopramide infusion, and her recovery after the washout of the drug further supports metoclopramide’s neurotoxicity in this case.
An interesting point is that adverse effects of metoclopramide can occur in the typical elderly population, mostly in forms of extrapyramidal disorders. However, metoclopramide-induced encephalopathy only occurred in patients with PD. This finding suggests that for such a rare adverse effect to happen, dopamine deficiency is a prerequisite to metoclopramide-induced encephalopathy, as shown in our patient. This case represents an additional example of metoclopramide-induced impaired mental status in degenerative parkinsonism and suggests that clinicians should be aware of such a complication.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.
Compliance with Ethical Standards
The institutional review board at St. Mary’s Hospital approved this case report (KC18ZESI0513).
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) and funded by the Ministry of Science, ICT and Future Planning (NRF-2017R1D1A1B06028086).
