Articles
- Page Path
- HOME > J Mov Disord > Volume 12(2); 2019 > Article
-
Original Article
Nonmotor and Dopamine Transporter Change in REM Sleep Behavior Disorder by Olfactory Impairment -
Jee-Young Lee1,2
 , Eun Jin Yoon3,4, Yu Kyeong Kim3,5
, Eun Jin Yoon3,4, Yu Kyeong Kim3,5 , Chae Won Shin6, Hyunwoo Nam1,2, Jae Min Jeong4,5, Han-Joon Kim5,6, Beomseok Jeon2,7
, Chae Won Shin6, Hyunwoo Nam1,2, Jae Min Jeong4,5, Han-Joon Kim5,6, Beomseok Jeon2,7 -
Journal of Movement Disorders 2019;12(2):103-112.
DOI: https://doi.org/10.14802/jmd.18061
Published online: May 30, 2019
1Department of Neurology, Seoul National University–Seoul Metropolitan Government Boramae Medical Center, Seoul, Korea
2Department of Neurology, Seoul National University College of Medicine, Seoul, Korea
3Department of Nuclear Medicine, Seoul National University–Seoul Metropolitan Government Boramae Medical Center, Seoul, Korea
4Institute of Radiation Medicine, Seoul National University Medical Research Center, Seoul National University, Seoul, Korea
5Department of Nuclear Medicine, Seoul National University College of Medicine, Seoul, Korea
6Department of Neurology, Kyung Hee University Medical Center, Seoul, Korea
7Department of Neurology and Movement Disorders Center, Seoul National University Hospital, Seoul, Korea
- Corresponding author: Jee-Young Lee, MD, PhD Department of Neurology, Seoul National University–Seoul Metropolitan Government Boramae Medical Center, 20 Boramae-ro 5-gil, Dongjak-gu, Seoul 07061, Korea / Tel: +82-2-870-2476 / Fax: +82-2-831-2826 / E-mail: wieber04@snu.ac.kr
- Corresponding author: Yu Kyeong Kim, MD, PhD Department of Nuclear Medicine, Seoul National University–Seoul Metropolitan Government Boramae Medical Center, 20 Boramae-ro 5-gil, Dongjakgu, Seoul 07061, Korea / Tel: +82-2-870-2591 / Fax: +82-2-870-3863 / E-mail: yk3181@snu.ac.kr
Copyright © 2019 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objective
- It is unclear whether the decline in dopamine transporters (DAT) differs among idiopathic rapid eye movement sleep behavior disorder (iRBD) patients with different levels of olfactory impairment. This study aimed to characterize DAT changes in relation to nonmotor features in iRBD patients by olfactory loss.
-
Methods
- This prospective cohort study consisted of three age-matched groups: 30 polysomnography-confirmed iRBD patients, 30 drug-naïve Parkinson’s disease patients, and 19 healthy controls without olfactory impairment. The iRBD group was divided into two groups based on olfactory testing results. Participants were evaluated for reported prodromal markers and then underwent 18F-FP-CIT positron emission tomography and 3T MRI. Tracer uptakes were analyzed in the caudate, anterior and posterior putamen, substantia nigra, and raphe nuclei.
-
Results
- Olfactory impairment was defined in 38.5% of iRBD patients. Mild parkinsonian signs and cognitive functions were not different between the two iRBD subgroups; however, additional prodromal features, constipation, and urinary and sexual dysfunctions were found in iRBD patients with olfactory impairment but not in those without. Tracer uptake showed significant group differences in all brain regions, except the raphe nuclei. The iRBD patients with olfactory impairment had uptake reductions in the anterior and posterior putamen, caudate, and substantia nigra (p < 0.016 in all, adjusted for age), which ranged from 0.6 to 0.8 of age-normative values. In contrast, those without olfactory impairment had insignificant changes in all regions ranging above 0.8.
-
Conclusion
- There was a clear distinction in DAT loss and nonmotor profiles by olfactory status in iRBD.
- Study participants
- This study was approved by the Institutional Review Board of Boramae Medical Center (16-2013-101). All participants gave informed consent prior to participation in the study in accordance with the Declaration of Helsinki.
- A cohort was consecutively constructed between September 2013 and August 2015. The iRBD group consisted of patients aged 60 to 80 years old. The diagnosis of RBD was made according to the International Classification of Sleep Disorders, 2nd edition, criteria by a sleep disorders specialist (H.W.N.) and confirmed by video polysomnography. The drug-naïve PD patients were consecutively recruited among those who were newly diagnosed during the study period in accordance with the UK PD brain bank criteria. All PD patients had a prolonged history of probable RBD with an RBD screening questionnaire score ≥ 5 [15,16]. The healthy control group was recruited during the study period from those who visited the same hospitals for health check-ups. The PD and control groups were recruited to be matched for the ages of the iRBD group.
- Exclusion criteria for all three groups included white matter changes greater than grade I small vessel disease, space-occupying lesions, structural lesions revealed on conventional brain MRI, history of nasal or sinus diseases, history of depression or other psychiatric illness, presence of other neurological diseases, presence of dementia or symptoms of cognitive fluctuation and visual hallucination suggesting dementia with Lewy bodies (DLB). For this, all subjects underwent a validated neuropsychological test battery (Seoul Neuropsychological Screening Battery; Human Brain Research & Consulting, Seoul, Korea) [17] during the screening period. We also excluded patients taking antipsychotics or antidepressants that affect the dopamine and serotonin systems due to the possibility of alterations in tracer uptake.
- Clinical evaluations
- Demographic data, information on RBD symptom duration, and age at disease onset were recorded. Olfactory function was evaluated by using the brief smell identification test (B-SIT) [18,19] and the buthanol threshold test (BTT) [20,21]. We defined olfactory impairment in our patients only if individuals consistently showed abnormal BTT (< 6) and more than a mild degree of impairment in B-SIT (scores < 6). Parkinsonian nonmotor and motor symptoms were assessed by using the Movement Disorders Society Task Force-revised Unified PD Rating Scale (MDSUPDRS; evaluations performed by a certified movement specialist J.Y.L.) and the Non-Motor Symptoms Scale (NMSS) [22]. Mild parkinsonian motor symptoms were categorized into four domains based on the MDS-UPDRS part III: 1) rigidity (item 3.3), 2) limb and body bradykinesia (items 3.4–3.8 and 3.14), 3) axial symptoms (items 3.1, 3.2, and 3.9–3.13), and 4) rest tremor (items 3.17 and 3.18). General cognitive status was determined by using the Korean version of the Mini-Mental Status Examination and Geriatric Depression Scale-15, and cognitive functions were further evaluated using the digit span backward test, Korean color Word Stroop Test, Trail Making Test (TMT) A and B, Controlled Oral Word Association Test, Seoul Verbal Learning Test (SVLT), Rey Complex Figure Test (RCFT) copy, and Korean version of the Boston Naming Test.
- Image acquisition
- Before scanning, all subjects were confirmed to be in a drugnaïve state both for dopaminergic and serotonergic drugs. Each participant underwent PET (Philips Gemini TF-64 PET/CT scanner, Philips Healthcare, Best, the Netherlands). After a bolus injection of 185 MBq of 18F-FP-CIT, subjects waited for 2 hours and then underwent 10 minute-emission scans. After routine corrections for physical effects, images were reconstructed by applying a 3D row-action maximum-likelihood algorithm for 90 slices, each 2 mm thick, in a 128 × 128 matrix with corrections for attenuation and scatter [23]. Each participant also underwent 3T brain MRI (Philips Achieva, Philips Healthcare). The acquisition parameters for the volumetric T1 images were as follows: repetition time/echo time = 9.9/4.6, flip angle of 8.0°, 1 mm slice thickness, image matrix of 224 × 224 × 180, and voxel size of 0.98 × 0.98 × 1 mm3.
- Analysis of 18F-FP-CIT uptakes
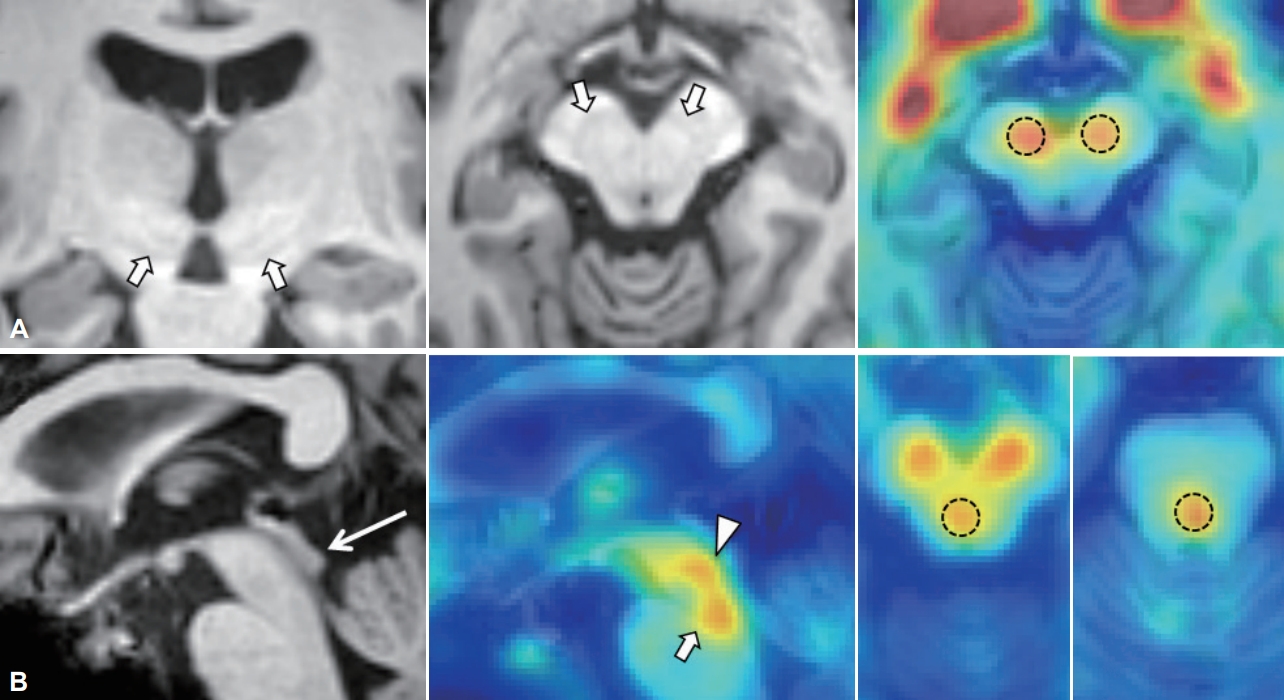
- In addition to basal ganglia structures, we included raphe nuclei as a reference for serotonin transporter uptake by considering that FP-CIT has cross-affinity to serotonin transporters. Anatomical boundaries for the bilateral caudate, putamen, and cerebellum (as a reference) were obtained automatically on T1-weighted MR images by using the segmentation tool FIRST integrated in FSL version 5.0.2 (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/first). The putamen for each hemisphere was divided into anterior and posterior halves along its longitudinal axis [24]. For the substantia nigra and the dorsal and median raphe nuclei, circular-shaped, fixed-size regions of interest were set manually on PET-overlaid MR images by using ITK-SNAP software (version 3.6.0-RC1; www.itksnap.org) (Figure 1). For raphe nuclei, we used the inferior colliculus as a landmark following the protocol reported previously [25,26]. After identifying the anatomical locations, we placed the regions of interest on 1 mm-thickness transverse slices centered on the maximal PET signals. The volume of interest was 46.3 mm3 for the subthalamic nucleus, 169.8 mm3 for the substantia nigra, and 127.3 mm3 for each raphe nucleus. The specific uptake of 18F-FP-CIT in the target volume of interest was calculated as follows: specific uptake of 18F-FP-CIT = [(average count in target/average count in cerebellum) - 1].
- Statistical analysis
- Initial analysis included comparisons of clinical variables and tracer uptakes in each region among the PD, iRBD, and control groups. Subsequent analysis compared the two subgroups of iRBD patients with the control subjects. In the analysis of 18FFP-CIT uptake according to the iRBD subgroups, we used linear regression analysis with age as a cofactor after confirming the fulfilment of the normality assumption in our FP-CIT data distribution. For other analyses, we used ANOVA, nonparametric Kruskal-Wallis, and Mann-Whitney U tests for continuous variables and chi-square or Fisher’s exact tests for categorical variables. In the post hoc analysis of intergroup comparisons, the Bonferroni correction was applied for multiple comparisons. Finally, we conducted correlation analysis between the severity of nonmotor symptoms and regional 18F-FP-CIT uptake in the iRBD group by applying partial correlation analysis controlling for age. We used SPSS software (version 19.0; IBM Corp., Armonk, NY, USA) for all statistical analyses, and the significance level was set at 0.05 (two-tailed).
MATERIALS & METHODS
- Of the 33 eligible PD patients, 31 iRBD patients, and 20 controls, 3 PD, 1 iRBD, and 1 control subjects were excluded from the study for the following reasons: structural abnormalities on MRI, failure of image acquisition, or refusal to scan. The baseline characteristics of the cohorts are summarized in Table 1. There were no differences in age and sex distributions, general cognition status, or depression scores among the three groups.
- Clinical assessments in three cohorts
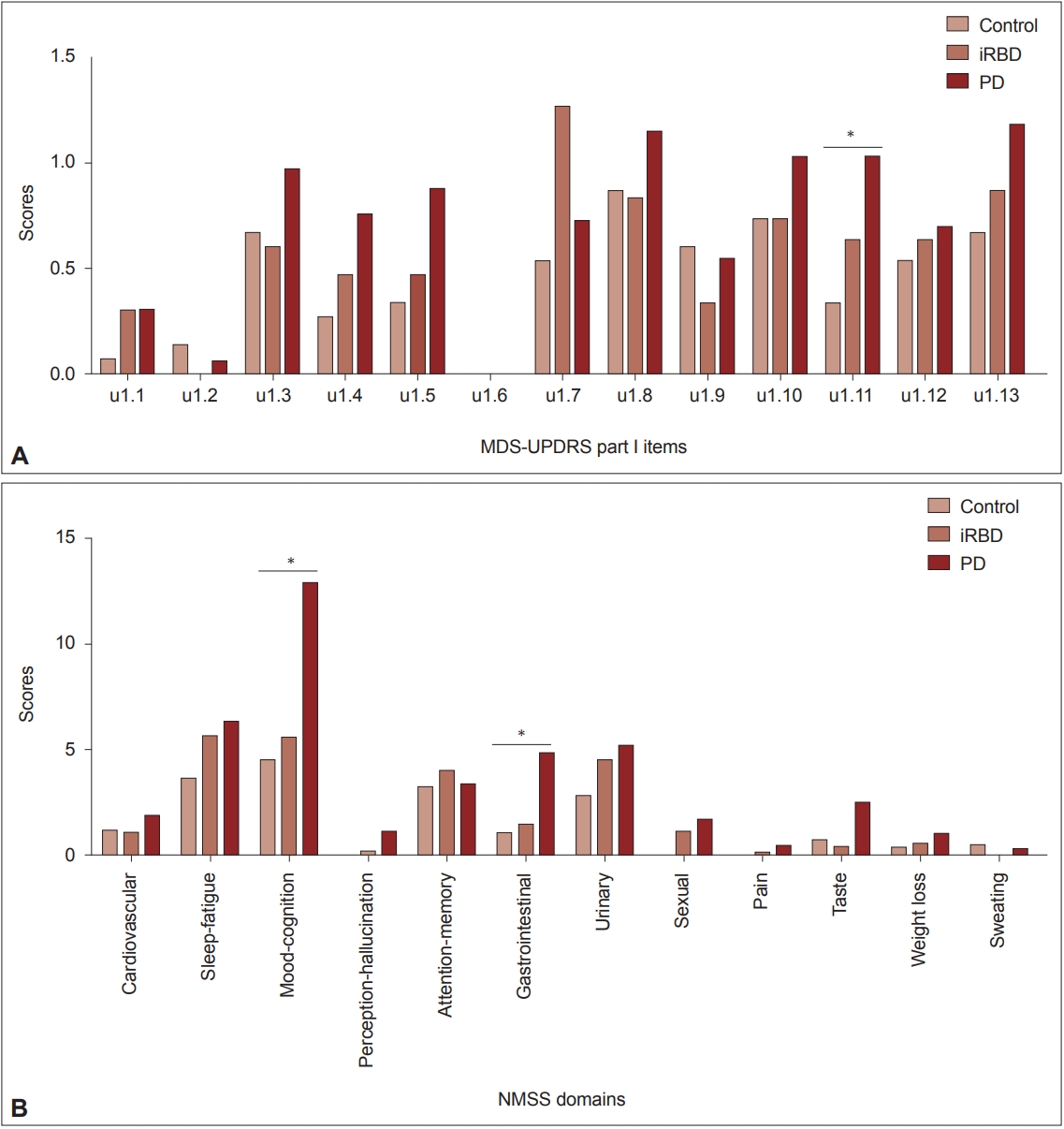
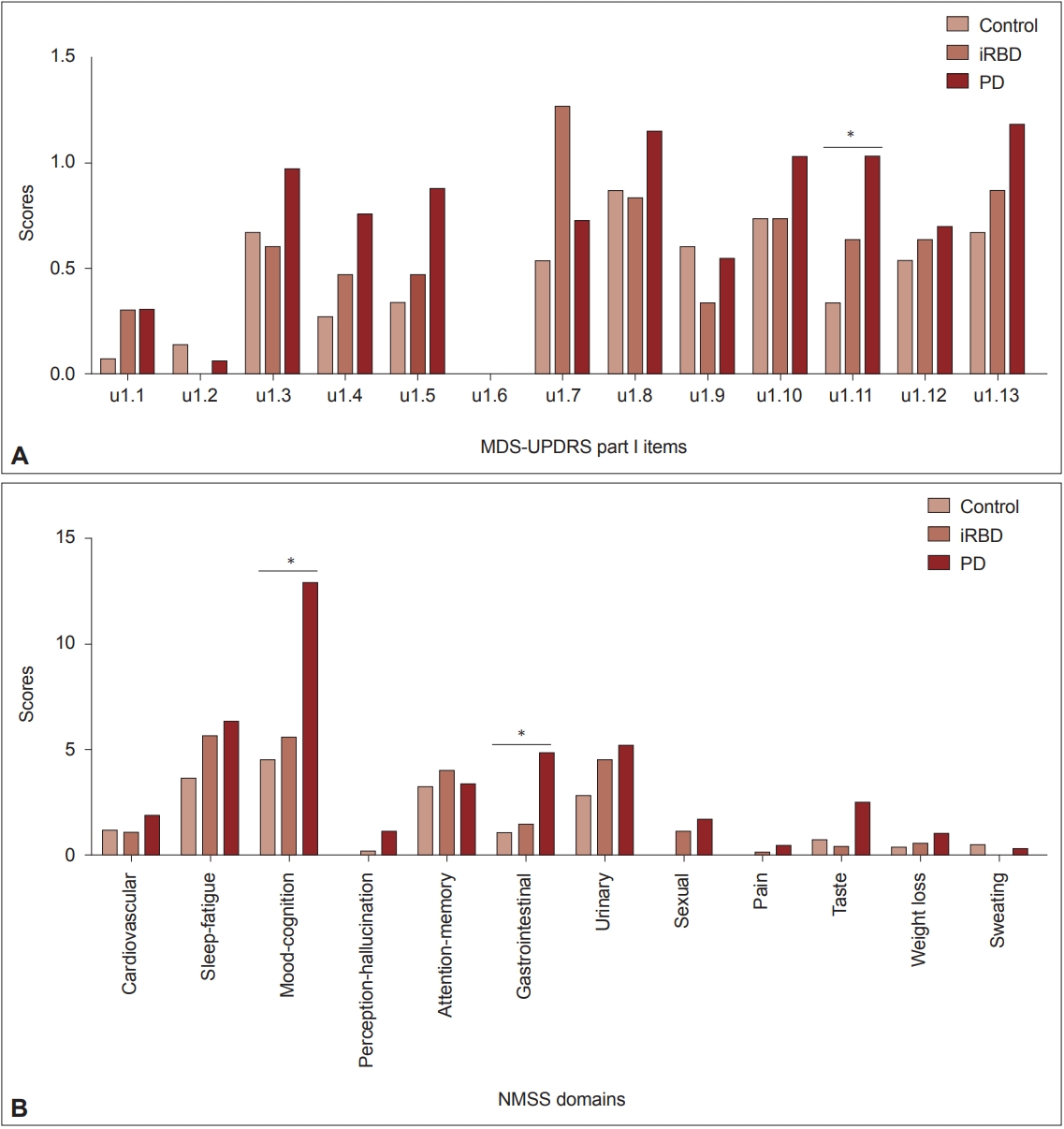
- Olfactory impairment was detected in 38.5% of iRBD patients and 50% of PD patients (Table 1). Because olfactory loss may be a risk factor for neurodegenerative diseases other than Lewy body diseases, we intentionally included only normal controls without hyposmia during the screening period. Analysis of nonmotor symptom items in the MDS-UPDRS part I revealed a significant group difference only in constipation (item 1.11). The mean scores of item 1.11 showed a linearly increasing tendency from the control to PD groups (p = 0.027). A similar but insignificant trend was found in items indicating anxiety (item 1.4), apathy (item 1.5), orthostatic dizziness (item 1.12), and fatigue (item 1.13) (Figure 2A). When we compared nonmotor symptoms by NMSS domain, the Mood/cognition and Gastrointestinal tract domains showed trends of linearly increasing scores from the control to PD groups (p = 0.043 and 0.010, respectively). Similar but insignificant trends were observed in the domains of sleep/fatigue, urinary, and sexual dysfunctions (Figure 2B). For the comparison of cognitive functions, we used z-scores of each cognitive test reflecting age and educational years. There were significant differences in the TMTB and SVLT recognition scores (p = 0.041 and 0.019, respectively) and a tendency of differences in the RCFT copy (p = 0.086), in which the PD and iRBD groups showed lower scores than the control group. When we defined impairment in each cognitive test if the z-score was below 1.5 standard deviations (SD) of normative values, the PD and iRBD groups showed a higher frequency of impairments in the TMT-B and RCFT copy compared to the control group (N, numbers of subjects with impairment = 8, 9, and 0 for PD, RBD, and controls, p = 0.034, and n = 7, 14, and 3, p = 0.029, respectively). Details of the neuropsychological test results are depicted in Supplementary Table 1 (in the online-only Data Supplement).
- 18F-FP-CIT uptakes in three cohorts
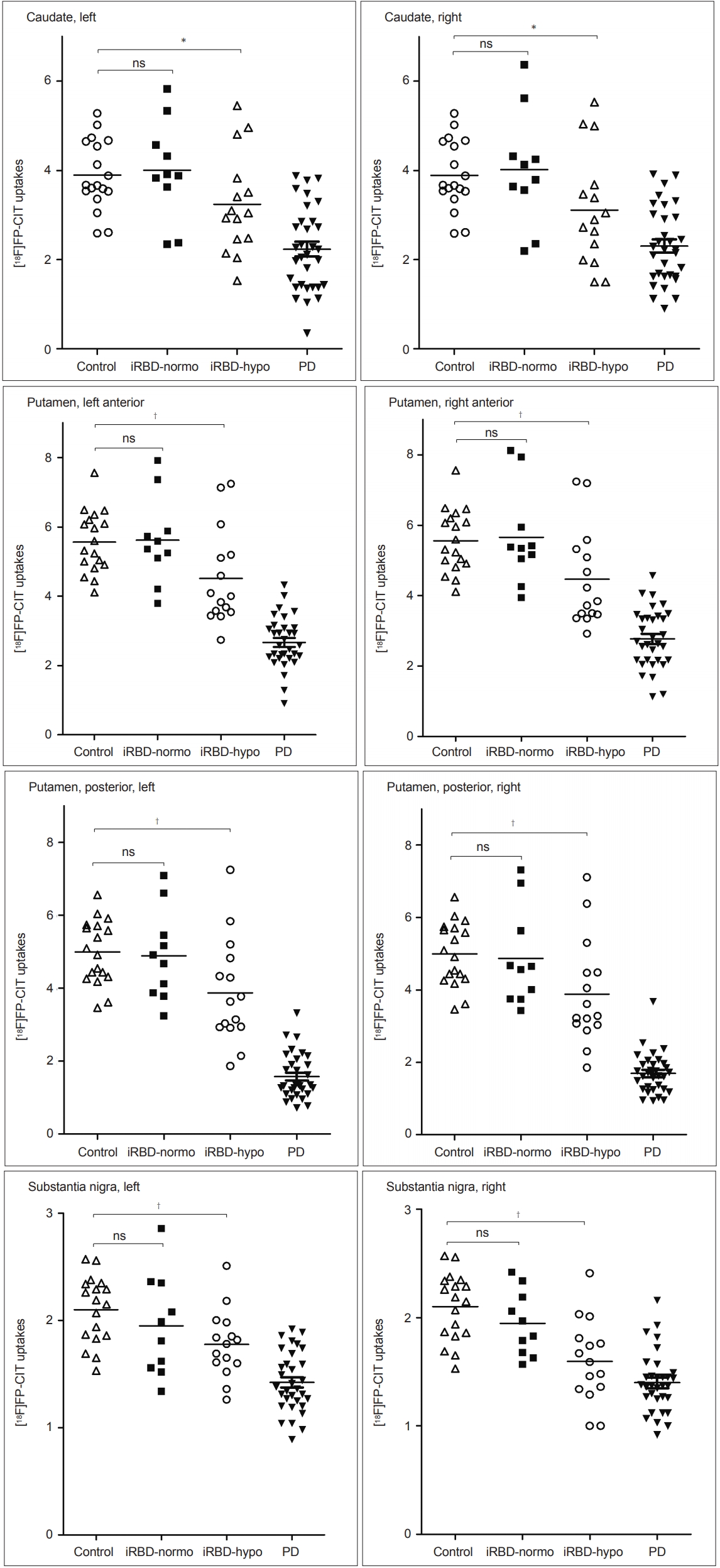
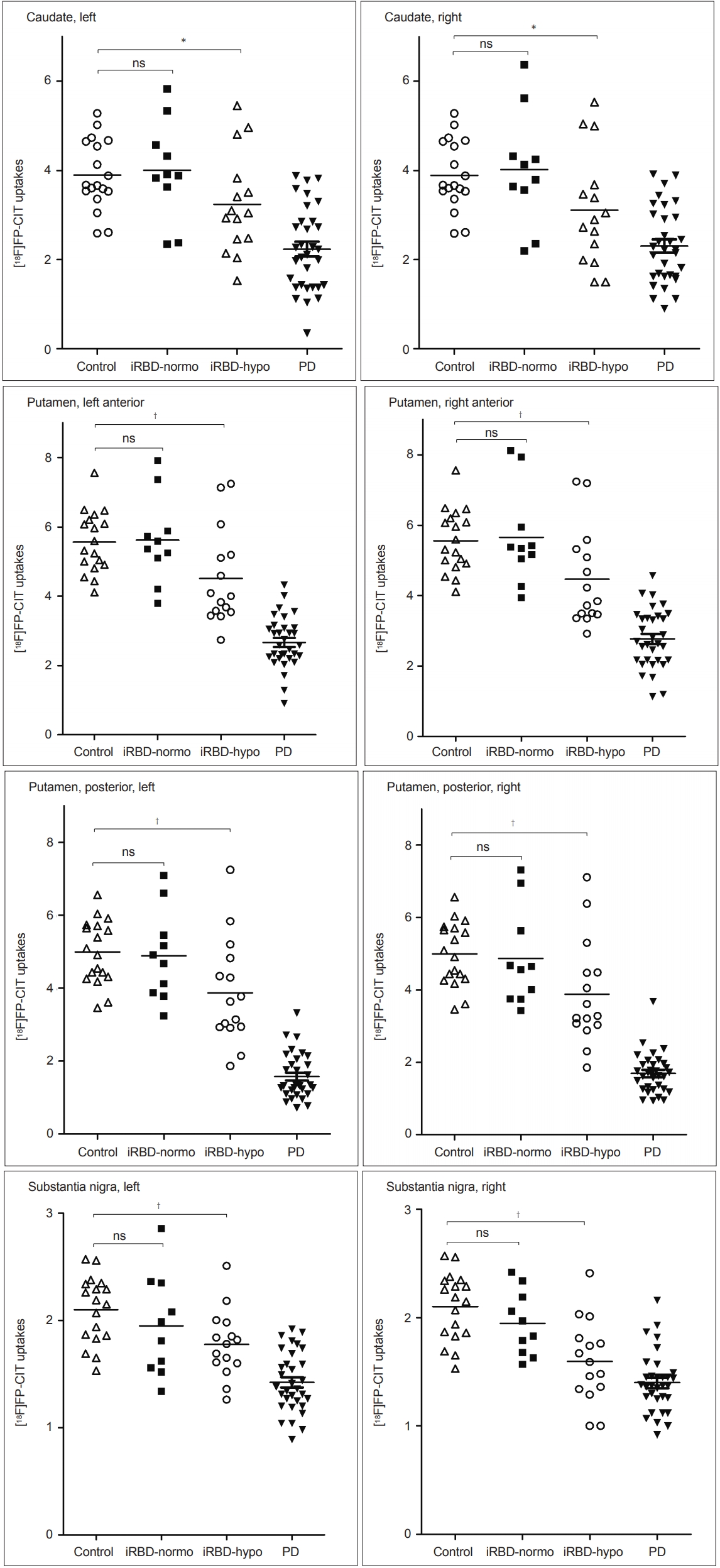
- There was a prominent decrease in the DAT density measured by 18F-FP-CIT uptake in the PD group compared to those in the iRBD and control groups in all regions, but uptake was indistinguishable in the raphe nuclei among the three groups and between any two groups. The mean 18F-FP-CIT uptake of the iRBD group was intermediate between those of the PD and control groups (Table 2). In the post hoc comparisons with controls, the iRBD group had reduced 18F-FP-CIT uptake in the left posterior putamen (p = 0.014) and the bilateral substantia nigra (p = 0.008 and 0.005).
- Analysis in iRBD patients with and without olfactory impairment
- Demographic and clinical features, including the duration of RBD, the MDS-UPDRS total, part 2 and 3 scores, total NMSS score, K-MMSE, and GDS scores of the two iRBD subgroups, were similar between the two iRBD subgroups (for all p > 0.05, Supplementary Table 2 in the online-only Data Supplement), but the iRBD patients with olfactory impairment tended to be male and to have a high MDS-UPRDS part I score (p = 0.058 and 0.060, respectively) (Supplementary Table 2 in the online-only Data Supplement).
- Nonmotor, cognition, and parkinsonian symptoms
- In the analysis of nonmotor symptoms, we found a significant difference only in constipation (i.e., item 1.11) of the MDS-UPDRS part I and in the gastrointestinal, urinary, and sexual symptom domains of the NMSS. The scores of these nonmotor symptoms were high in the iRBD-olfactory impairment group, but the scores in the iRBD without olfactory impairment group were indifferent from those in the control group (Table 3). Cognitive test scores and mild parkinsonian sign scores were not significantly different between the two iRBD subgroups (Supplementary Table 2 in the online-only Data Supplement). However, the TMT-B score tended to be lower (-2.03 ± 2.68 vs. -0.66 ± 1.34, p = 0.388), and the bradykinesia score tended to be higher in the olfactory impairment group than in the no impairment group (3.56 ± 2.64 vs. 2.88 ± 3.52, p = 0.162), but the difference was not statistically significant.
- Dopamine transporter reductions
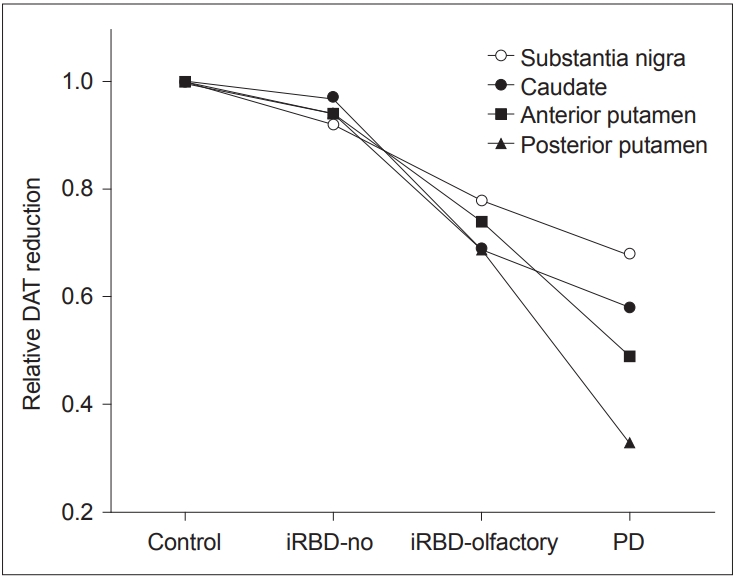
- When we analyzed the DAT PET data, the iRBD with olfactory impairment group showed significant reductions in 18FFP-CIT uptake in the caudate nucleus, anterior and posterior putamen, and substantia nigra compared to the HC group (Figure 3). In contrast, the iRBD without olfactory impairment group showed no regions of significantly reduced 18F-FP-CIT uptake (Figure 3). Relative DAT reductions averaged for both hemispheres in each iRBD subgroup in comparison to the HC and PD groups are depicted in Figure 4. DAT densities with reference to age-adjusted normative mean values were all above 0.8 in iRBD patients without olfactory impairment, whereas those ranged from 0.6 to 0.8 in all regions in iRBD patients with impairment (Figure 4).
- When we conducted correlation analysis in iRBD patients for DAT with nonmotor symptoms, a significant negative correlation was detected between the constipation score and 18F-FPCIT uptakes in the bilateral anterior and posterior putamen and substantia nigra (correlation coefficient ranged from -0.260 to -0.358, p < 0.05 for all, after controlling for age by partial correlation analysis). For other nonmotor symptoms and cognitive function scores, there were no significant correlations with DAT uptakes.
RESULTS
- The present study revealed features of DAT changes in relation to olfactory impairment and other prodromal features that were assessed systematically in our iRBD cohort.
- As shown in Figure 2, a trend of increasing severity from normal to disease conditions was observed in several nonmotor symptoms, including anxiety and apathy (or mood-cognition), constipation (gastrointestinal), some autonomic dysfunction (urinary, sexual, orthostatic dizziness), and fatigue. Although statistical significance was only detected in the gastrointestinal and mood-cognition symptoms, these nonmotor symptoms might be more disease-related than those without such trends. As reported in a large iRBD cohort [27], there was a close relationship between hyposmia and constipation in our cohort. The constipation symptom score increased from normal to disease conditions, and that score correlated with the DAT uptake reduction. Follow-up analysis of our cohort is needed to reveal the temporal relationship between the emergence of nonmotor and motor parkinsonian features and the DAT reduction in iRBD.
- By performing a quantitative assessment of DAT, we could clearly show the level of DAT reductions in the iRBD patients with olfactory impairment at 0.6–0.8 of age-adjusted normal values. There was also a distinctive pattern of DAT reduction in the putamen and caudate between the iRBD with olfactory impairment and drug-naïve PD groups. In the PD group, the DAT reduction in the posterior putamen was large, whereas that in the caudate was relatively small. In contrast, the DAT reductions in both the posterior putamen and the caudate were similar in the iRBD with olfactory impairment group. Therefore, it is suggested that neurodegeneration in iRBD patients with hyposmia may not be confined to a motor part of the basal ganglia. Combining DAT data with a high frequency of autonomic dysfunction and with the fact that common parkinsonism appearing in iRBD patients is known to be postural instability gait disturbance (PIGD) type [28], it is necessary to follow-up with this cohort to confirm whether our hyposmic iRBD patients will eventually develop PIGD-type poor prognostic PD or DLB-type Lewy body disease.
- We showed DAT reduction in the substantia nigra as well as in the striatum in our iRBD patients with olfactory impairment as well as in PD patients. The greatest statistical significance found in both substantia nigra might be affected by the variance difference in DAT data between the striatum and nigra. It is perceived that nigral neuronal loss starts at the distal axons [29], and thus, greater variability in the striatum was expected. On the other hand, DAT regulation in the nigra in PD was shown in a pathological study, as there may be a shift into neurons that express a lower level of DAT [10]. However, studies are needed to reveal nigral DAT regulation in early stage PD, and further studies using a specific DAT tracer should be undertaken to replicate our findings.
- Most of the 18F-FP-CIT uptake in raphe nuclei reflects serotonin transporters in the serotonergic cell bodies [28]. We included raphe nuclei in this analysis as a reference region for serotonergic binding because of the cross-affinity of FP-CIT to the serotonin transporter. Our study revealed that both raphe nuclei uptakes were not different among the groups. As in our study, preservation of the serotonergic system in early PD has been observed with tracers binding to serotonin transporters [30,31]. However, compared to serotonin receptor imaging, serotonin transporter uptake may not provide a robust correlation with serotonergic function in the brain [32]. Thus, serotonergic involvement in iRBD needs to be further investigated in studies that use tracers binding to postsynaptic receptors.
- Our data need to be interpreted with caution. First, we need to confirm the relationship between the nonmotor feature and regional neurodegenerative changes through a longitudinal follow-up. Second, the manually driven regions of interest for the substantia nigra might have reduced the power of detecting neurodegenerative changes because it had to be based on the axial MRI images, although the configurations of these structures are oblique. Third, the possibility that some of our iRBD patients will develop a neurodegenerative disease other than PD was not excluded. However, hyposmia is a specific premotor sign of Lewy body diseases rather than multiple system atrophy (MSA) [33], and our iRBD patients without olfactory impairment did not have symptoms suggesting autonomic dysfunctions or neurological signs of MSA.
- This study demonstrated that hyposmia can predict the level of DAT reduction and the presence of prodromal nonmotor features in iRBD subjects. Longitudinal follow-up of our cohort would help to reveal whether neurodegeneration differs by hyposmia over time in iRBD and to elucidate the temporal relationship between the emergence of clinical features and DAT loss in iRBD.
DISCUSSION
Supplementary Materials
Supplementary Table 1.
Supplementary Table 2
- We give our sincere and honest appreciation to our patients, volunteers, and caregivers for their enthusiastic participation in this study.
- This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (No. NRF-2014M3C7A1046042, 2016R1D1A1B03936159, and 2018R1C1B3008971).
Acknowledgments
| Characteristics | Drug-naïve PD | iRBD | Healthy controls | p-value* |
|---|---|---|---|---|
| Age, yrs | 69.2 ± 7.0 | 70.5 ± 5.9 | 70.1 ± 4.8 | 0.703 |
| Sex, F/M (n) | 14/19 | 14/17 | 12/7 | 0.323† |
| Duration of RBD, yrs | 4.8 ± 3.9 | 4.3 ± 3.0 | - | 0.717 |
| Duration of PD, yrs | 1.1 ± 0.5 | - | - | |
| Hoehn & Yahr stage | 1.6 ± 0.5 | - | - | |
| K-MMSE score | 27.1 ± 2.6 | 27.6 ± 2.1 | 28.1 ± 1.8 | 0.234 |
| GDS-15 score | 6.9 ± 3.6 | 6.0 ± 3.1 | 5.5 ± 3.8 | 0.346 |
| MDS-UPDRS score | ||||
| Part I | 9.3 ± 7.2 | 7.1 ± 3.5 | 5.7 ± 3.8 | 0.081 |
| Part II | 8.6 ± 6.5 | 3.3 ± 3.3 | 2.6 ± 2.9 | < 0.001§ |
| Part III | 25.0 ± 11.9 | 5.8 ± 4.9 | 0.7 ± 1.3 | < 0.001§,‖ |
| Hyposmia, % | 50.0 | 38.5 | 0‡ | 0.002†,‖,¶ |
| NMSS score, total | 41.5 ± 50.7 | 24.3 ± 20.1 | 19.5 ± 11.6 | 0.069 |
Data are shown as the mean ± standard deviation unless otherwise indicated.
* comparison among the three groups by the ANOVA test unless otherwise specified,
† comparison among the three groups by the chi-square test,
‡ only healthy subjects who were not confirmed to have hyposmia were included in the control group in this cohort study (see details in the Materials and Methods section),
§ significant difference in post hoc comparisons between the PD and each of the two other groups (p < 0.016),
‖ significant difference in post hoc comparison between the iRBD and control groups (p < 0.016),
¶ significant difference in post hoc comparison between the PD and control groups (p < 0.016).
PD: Parkinson’s disease, iRBD: idiopathic rapid eye movement sleep behavior disorder, K-MMSE: Korean version of Mini-Mental Status Exam, GDS: Geriatric Depression Scale, MDS-UPDRS: Movement Disorders Society Task Force-revised Unified PD Rating Scale, NMSS: Non-Motor Symptom Scale.
| Regions | Drug-naïve PD | iRBD | Healthy controls | p-value* |
|---|---|---|---|---|
| Caudate nucleus | ||||
| Left | 2.23 ± 0.93 | 3.46 ± 1.10 | 3.89 ± 0.78 | < 0.001 |
| Right | 2.31 ± 0.85 | 3.40 ± 1.23 | 3.92 ± 0.85 | < 0.001 |
| Putamen, anterior | ||||
| Left | 2.67 ± 0.74 | 4.91 ± 1.30 | 5.56 ± 0.89 | < 0.001 |
| Right | 2.77 ± 0.84 | 4.90 ± 1.35 | 5.65 ± 0.88 | < 0.001 |
| Putamen, posterior | ||||
| Left | 1.57 ± 0.62 | 4.23 ± 1.36 | 4.99 ± 0.88 | < 0.001† |
| Right | 1.69 ± 0.56 | 4.24 ± 1.39 | 4.90 ± 0.86 | < 0.001 |
| Substantia nigra | ||||
| Left | 1.42 ± 0.28 | 1.84 ± 0.37 | 2.10 ± 0.31 | < 0.001† |
| Right | 1.40 ± 0.28 | 1.74 ± 0.38 | 2.02 ± 0.28 | < 0.001† |
| Raphe nucleus | ||||
| Dorsal | 1.37 ± 0.44 | 1.36 ± 0.46 | 1.48 ± 0.40 | 0.625 |
| Median | 1.33 ± 0.42 | 1.27 ± 0.42 | 1.30 ± 0.35 | 0.853 |
Data are shown as the mean ± standard deviation.
* comparison among the three groups of PD, iRBD, and controls by the ANOVA test,
† significant difference between iRBD and control groups by post hoc analysis (p = 0.008 and 0.005 for the left and right substantia nigra, and p = 0.014 for the left posterior putamen).
PD: Parkinson’s disease, iRBD: idiopathic rapid eye movement sleep behavior disorder.
|
iRBD |
Healthy controls | p-value* | ||
|---|---|---|---|---|
| Olfactory impairment | No olfactory impairment | |||
| MDS-UPDRS I.11 (constipation) | 1.08 ± 0.64 | 0.08 ± 0.29 | 0.33 ± 0.62 | < 0.001†,‡ |
| NMSS domain 6 (gastrointestinal tract) | 2.57 ± 2.03 | 0.25 ± 0.45 | 1.00 ± 2.27 | 0.002†,‡ |
| NMSS domain 7 (urinary) | 6.00 ± 5.73 | 3.75 ± 3.94 | 2.80 ± 1.90 | 0.021†,‡ |
| NMSS domain 8 (sexual function) | 3.00 ± 3.92 | 0.25 ± 1.00 | 0.00 ± 0.00 | 0.023† |
Data are shown as the mean ± standard deviation.
* comparison among the three groups by the Kruskal-Wallis test,
† significant difference between the iRBD-olfactory impairment and control groups by the Mann-Whitney U test (p < 0.016),
‡ significant difference between the two iRBD subgroups by the Mann-Whitney U test (p < 0.016). There was no significant difference between the iRBD-no olfactory impairment and control groups in any of the items and domains investigated.
iRBD: idiopathic rapid eye movement sleep behavior disorder, MDS-UPDRS: Movement Disorders Society Task Force-revised Unified Parkinson’s Disease Rating Scale, NMSS: Non-Motor Symptom Scale.
- 1. Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, et al. MDS research criteria for prodromal Parkinson’s disease. Mov Disord 2015;30:1600–1611.ArticlePubMed
- 2. Postuma RB, Gagnon JF, Bertrand JA, Genier Marchand D, Montplaisir JY. Parkinson risk in idiopathic REM sleep behavior disorder: preparing for neuroprotective trials. Neurology 2015;84:1104–1113.ArticlePubMedPMC
- 3. Postuma RB, Gagnon JF, Vendette M, Montplaisir JY. Markers of neurodegeneration in idiopathic rapid eye movement sleep behaviour disorder and Parkinson’s disease. Brain 2009;132(Pt 12):3298–3307.ArticlePubMedPDF
- 4. Mahlknecht P, Iranzo A, Högl B, Frauscher B, Müller C, Santamaría J, et al. Olfactory dysfunction predicts early transition to a Lewy body disease in idiopathic RBD. Neurology 2015;84:654–658.ArticlePubMed
- 5. Jennings D, Siderowf A, Stern M, Seibyl J, Eberly S, Oakes D, et al. Imaging prodromal Parkinson disease: the Parkinson associated risk syndrome study. Neurology 2014;83:1739–1746.ArticlePubMedPMC
- 6. Iranzo A, Serradell M, Vilaseca I, Valldeoriola F, Salamero M, Molina C, et al. Longitudinal assessment of olfactory function in idiopathic REM sleep behavior disorder. Parkinsonism Relat Disord 2013;19:600–604.ArticlePubMed
- 7. Postuma RB, Gagnon JF, Vendette M, Desjardins C, Montplaisir JY. Olfaction and color vision identify impending neurodegeneration in rapid eye movement sleep behavior disorder. Ann Neurol 2011;69:811–818.ArticlePubMed
- 8. Postuma RB, Iranzo A, Hogl B, Arnulf I, Ferini-Strambi L, Manni R, et al. Risk factors for neurodegeneration in idiopathic rapid eye movement sleep behavior disorder: a multicenter study. Ann Neurol 2015;77:830–839.ArticlePubMedPMC
- 9. Iranzo A, Valldeoriola F, Lomeña F, Molinuevo JL, Serradell M, Salamero M, et al. Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol 2011;10:797–805.ArticlePubMed
- 10. Joyce JN, Smutzer G, Whitty CJ, Myers A, Bannon MJ. Differential modification of dopamine transporter and tyrosine hydroxylase mRNAs in midbrain of subjects with Parkinson’s, Alzheimer’s with parkinsonism, and Alzheimer’s disease. Mov Disord 1997;12:885–897.ArticlePubMed
- 11. Whone AL, Moore RY, Piccini PP, Brooks DJ. Plasticity of the nigropallidal pathway in Parkinson’s disease. Ann Neurol 2003;53:206–213.ArticlePubMed
- 12. Brown CA, Karimi MK, Tian L, Flores H, Su Y, Tabbal SD, et al. Validation of midbrain positron emission tomography measures for nigrostriatal neurons in macaques. Ann Neurol 2013;74:602–610.ArticlePubMed
- 13. Karimi M, Tian L, Brown CA, Flores HP, Loftin SK, Videen TO, et al. Validation of nigrostriatal positron emission tomography measures: critical limits. Ann Neurol 2013;73:390–396.ArticlePubMedPMC
- 14. Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J Neurosci 1996;16:436–447.ArticlePubMedPMC
- 15. Lim JS, Shin SA, Lee JY, Nam H, Lee JY, Kim YK. Neural substrates of rapid eye movement sleep behavior disorder in Parkinson’s disease. Parkinsonism Relat Disord 2016;23:31–36.ArticlePubMed
- 16. Stiasny-Kolster K, Mayer G, Schäfer S, Möller JC, Heinzel-Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire--a new diagnostic instrument. Mov Disord 2007;22:2386–2393.ArticlePubMed
- 17. Kim HJ, Im K, Kwon H, Lee JM, Kim C, Kim YJ, et al. Clinical effect of white matter network disruption related to amyloid and small vessel disease. Neurology 2015;85:63–70.ArticlePubMedPMC
- 18. Lee JE, Cho KH, Ham JH, Song SK, Sohn YH, Lee PH. Olfactory performance acts as a cognitive reserve in non-demented patients with Parkinson’s disease. Parkinsonism Relat Disord 2014;20:186–191.ArticlePubMed
- 19. Ross GW, Petrovitch H, Abbott RD, Tanner CM, Popper J, Masaki K, et al. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann Neurol 2008;63:167–173.ArticlePubMed
- 20. Yang KH, Kim IT, Park YM, Min YG. Measurement of olfactory threshold in normal Korean adults with combined use of bounded CCCRC test and Step method. J Rhinol 1997;4:13–17.
- 21. Kim BG, Oh JH, Choi HN, Park SY. Simple assessment of olfaction in patients with chronic rhinosinusitis. Acta Otolaryngol 2015;135:258–263.ArticlePubMed
- 22. Chaudhuri KR, Martinez-Martin P, Brown RG, Sethi K, Stocchi F, Odin P, et al. The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: results from an international pilot study. Mov Disord 2007;22:1901–1911.ArticlePubMed
- 23. Lee JY, Seo S, Lee JS, Kim HJ, Kim YK, Jeon BS. Putaminal serotonergic innervation: monitoring dyskinesia risk in Parkinson disease. Neurology 2015;85:853–860.ArticlePubMed
- 24. Morrish PK, Sawle GV, Brooks DJ. Regional changes in [18F]dopa metabolism in the striatum in Parkinson’s disease. Brain 1996;119(Pt 6):2097–2103.ArticlePubMedPDF
- 25. Son YD, Cho ZH, Choi EJ, Kim JH, Kim HK, Lee SY, et al. Individually differentiated serotonergic raphe nuclei measured with brain PET/MR imaging. Radiology 2014;272:541–548.ArticlePubMed
- 26. Son YD, Cho ZH, Kim HK, Choi EJ, Lee SY, Chi JG, et al. Glucose metabolism of the midline nuclei raphe in the brainstem observed by PET-MRI fusion imaging. Neuroimage 2012;59:1094–1097.ArticlePubMed
- 27. Aguirre-Mardones C, Iranzo A, Vilas D, Serradell M, Gaig C, Santamaría J, et al. Prevalence and timeline of nonmotor symptoms in idiopathic rapid eye movement sleep behavior disorder. J Neurol 2015;262:1568–1578.ArticlePubMedPDF
- 28. Iranzo A, Santamaria J, Tolosa E. Idiopathic rapid eye movement sleep behaviour disorder: diagnosis, management, and the need for neuroprotective interventions. Lancet Neurol 2016;15:405–419.ArticlePubMed
- 29. Saari L, Kivinen K, Gardberg M, Joutsa J, Noponen T, Kaasinen V. Dopamine transporter imaging does not predict the number of nigral neurons in Parkinson disease. Neurology 2017;88:1461–1467.ArticlePubMed
- 30. Politis M, Wu K, Loane C, Kiferle L, Molloy S, Brooks DJ, et al. Staging of serotonergic dysfunction in Parkinson’s disease: an in vivo 11C-DASB PET study. Neurobiol Dis 2010;40:216–221.ArticlePubMed
- 31. Albin RL, Koeppe RA, Bohnen NI, Wernette K, Kilbourn MA, Frey KA. Spared caudal brainstem SERT binding in early Parkinson’s disease. J Cereb Blood Flow Metab 2008;28:441–444.ArticlePubMed
- 32. Komorowski A, James GM, Philippe C, Gryglewski G, Bauer A, Hienert M, et al. Association of protein distribution and gene expression revealed by PET and post-mortem quantification in the serotonergic system of the human brain. Cereb Cortex 2017;27:117–130.ArticlePubMedPDF
- 33. Jecmenica-Lukic M, Poewe W, Tolosa E, Wenning GK. Premotor signs and symptoms of multiple system atrophy. Lancet Neurol 2012;11:361–368.ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Neuropsychological Changes in Isolated REM Sleep Behavior Disorder: A Systematic Review and Meta-analysis of Cross-sectional and Longitudinal Studies
Caterina Leitner, Giada D’Este, Laura Verga, Shady Rahayel, Samantha Mombelli, Marco Sforza, Francesca Casoni, Marco Zucconi, Luigi Ferini-Strambi, Andrea Galbiati
Neuropsychology Review.2024; 34(1): 41. CrossRef - Dopamine transporter positron emission tomography in patients with Alzheimer’s disease with Lewy body disease features
Sungwoo Kang, Seun Jeon, Young-gun Lee, Byoung Seok Ye
Neurobiology of Aging.2024; 134: 57. CrossRef - Imaging Procedure and Clinical Studies of [18F]FP-CIT PET
Changhwan Sung, Seung Jun Oh, Jae Seung Kim
Nuclear Medicine and Molecular Imaging.2024;[Epub] CrossRef - Validation of the REM behaviour disorder phenoconversion-related pattern in an independent cohort
Beatrice Orso, Pietro Mattioli, Eun-Jin Yoon, Yu Kyeong Kim, Heejung Kim, Jung Hwan Shin, Ryul Kim, Claudio Liguori, Francesco Famà, Andrea Donniaquio, Federico Massa, David Vállez García, Sanne K. Meles, Klaus L. Leenders, Agostino Chiaravalloti, Matteo
Neurological Sciences.2023; 44(9): 3161. CrossRef - Neurofilament light chain and cardiac MIBG uptake as predictors for phenoconversion in isolated REM sleep behavior disorder
Don Gueu Park, Ju Yeong Kim, Min Seung Kim, Mi Hee Kim, Young-Sil An, Jaerak Chang, Jung Han Yoon
Journal of Neurology.2023; 270(9): 4393. CrossRef - Longitudinal evolution of cortical thickness signature reflecting Lewy body dementia in isolated REM sleep behavior disorder: a prospective cohort study
Jung Hwan Shin, Heejung Kim, Yu Kyeong Kim, Eun Jin Yoon, Hyunwoo Nam, Beomseok Jeon, Jee-Young Lee
Translational Neurodegeneration.2023;[Epub] CrossRef - Brain olfactory‐related atrophy in isolated rapid eye movement sleep behavior disorder
Kyung Ah Woo, Heejung Kim, Eun Jin Yoon, Jung Hwan Shin, Hyunwoo Nam, Beomseok Jeon, Yu Kyeong Kim, Jee‐Young Lee
Annals of Clinical and Translational Neurology.2023; 10(12): 2192. CrossRef - Monoaminergic Degeneration and Ocular Motor Abnormalities in De Novo Parkinson's Disease
Kyung Ah Woo, Joo Hong Joun, Eun Jin Yoon, Chan Young Lee, Beomseok Jeon, Yu Kyeong Kim, Jee‐Young Lee
Movement Disorders.2023; 38(12): 2291. CrossRef - Altered cerebral perfusion and microstructure in advanced Parkinson’s disease and their associations with clinical features
Zhaoxi Liu, Yiwei Zhang, Han Wang, Dan Xu, Hui You, Zhentao Zuo, Feng Feng
Neurological Research.2022; 44(1): 47. CrossRef - Brain Neuroimaging of Rapid Eye Movement Sleep Behavior Disorder in Parkinson’s Disease: A Systematic Review
Rafail Matzaras, Kuangyu Shi, Artemios Artemiadis, Panagiotis Zis, Georgios Hadjigeorgiou, Axel Rominger, Claudio L.A. Bassetti, Panagiotis Bargiotas
Journal of Parkinson's Disease.2022; 12(1): 69. CrossRef - Odor Identification by Parkinson’s Disease Patients Tested by Using Sniffin’ Sticks versus Natural Spices
Florence Baert, Geertrui Vlaemynck, Jarissa Maselyne, Christophe Matthys, Seyed-Mohammad Fereshtehnejad
Parkinson's Disease.2022; 2022: 1. CrossRef - Brain Metabolic Correlates of Dopaminergic Denervation in Prodromal and Early Parkinson's Disease
Ryul Kim, Heejung Kim, Yu Kyeong Kim, Eun Jin Yoon, Hyun Woo Nam, Beomseok Jeon, Jee‐Young Lee
Movement Disorders.2022; 37(10): 2099. CrossRef - Longitudinal Changes in Isolated Rapid Eye Movement Sleep Behavior Disorder‐Related Metabolic Pattern Expression
Ryul Kim, Jee‐Young Lee, Yu Kyeong Kim, Heejung Kim, Eun Jin Yoon, Jung Hwan Shin, Dallah Yoo, Hyunwoo Nam, Beomseok Jeon
Movement Disorders.2021; 36(8): 1889. CrossRef - Parkinson Disease-Related Brain Metabolic Patterns and Neurodegeneration in Isolated REM Sleep Behavior Disorder
Jung Hwan Shin, Jee-Young Lee, Yu-Kyeong Kim, Eun Jin Yoon, Heejung Kim, Hyunwoo Nam, Beomseok Jeon
Neurology.2021;[Epub] CrossRef - Retina Thickness as a Marker of Neurodegeneration in Prodromal Lewy Body Disease
Jee‐Young Lee, Jeeyun Ahn, Sohee Oh, Joo Young Shin, Yu Kyeong Kim, Hyunwoo Nam, Beomseok Jeon
Movement Disorders.2020; 35(2): 349. CrossRef - Serum TNF-α and neurodegeneration in isolated REM sleep behavior disorder
Ryul Kim, Jee-Young Lee, Han-Joon Kim, Yu Kyeong Kim, Hyunwoo Nam, Beomseok Jeon
Parkinsonism & Related Disorders.2020; 81: 1. CrossRef - Longitudinal change in dopamine transporter availability in idiopathic REM sleep behavior disorder
Jung Hwan Shin, Jee-Young Lee, Yu-Kyeong Kim, Sung-A Shin, Heejung Kim, Hyunwoo Nam, Beomseok Jeon
Neurology.2020;[Epub] CrossRef
Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite