Dear Editor,
Parkinson’s disease (PD) is primarily caused by a loss of dopamine neurons in the substantia nigra (SN), where deposits of α-synuclein aggregates, Lewy bodies (LBs) and Lewy neurites are characteristic features [1]. The topographical distribution of the Lewy-related pathology (LB and Lewy neurites) determines the clinical presentation of PD; these pathologies in the SN are mostly associated with motor symptoms of PD, and their progression to the neocortex is generally linked to dementia [1-3]. In the late stage of PD, dementia is common and is even thought to be inevitable at a disease duration of 20 years per the Sydney multicenter study [4]. Herein, we describe a patient with typical PD without overt dementia for 18 years who showed brainstem-predominant Lewy-related pathology without other concomitant pathologies except for primary age-related tauopathy (PART).
A 61-year-old female presented with a 5-year duration of right-hand resting tremor. She had no prior medical history, nor did she have a family history of neurodegenerative disease. On examination, she described bilateral bradykinesia that was worse on the right side, rigidity on the right side, tremor in the right hand, and reduced arm swing during gait, which responded well to levodopa therapy. However, at age 63, a shorter and delayed response to the medication and dyskinesias occurred with a total daily levodopa equivalent dose of 525 mg, and at age 69, unpredictable motor fluctuations appeared, which were not well controlled with frequent administration of 60 mg of levodopa every hour. In terms of nonmotor symptoms, she had experienced dream enactment behavior a few years prior to the initial motor symptoms and had nonmotor pain during “off” periods, which appeared at age 69. Of note, she had no cognitive or neuropsychiatric problems throughout the course of the disease. Even at age 71, when she was in a state of being unable to walk without assistance due to worsening motor complications and chronic pain fluctuations, her cognition was well preserved with the absence of hallucinations, and there was no significant change in cognitive assessment results compared with the previous results. She was uneducated and illiterate, and her Korean Mini-Mental State Examination (K-MMSE) scores at age 67 were 21/30, and those scores at the late stage were 20/30, with points deducted mainly in the categories of language and calculation, followed by recall; her score in the Korean version of the Frontal Assessment Battery (FAB-K) at age 67 was 12/18. Brain MRI showed unremarkable findings except for mild diffuse cerebral atrophy with no significant atrophy in the medial temporal lobe (Supplementary Figure 1 in the online-only Data Supplement). At the age of 74, 18 years after the onset of symptoms, she died from aspiration pneumonia.
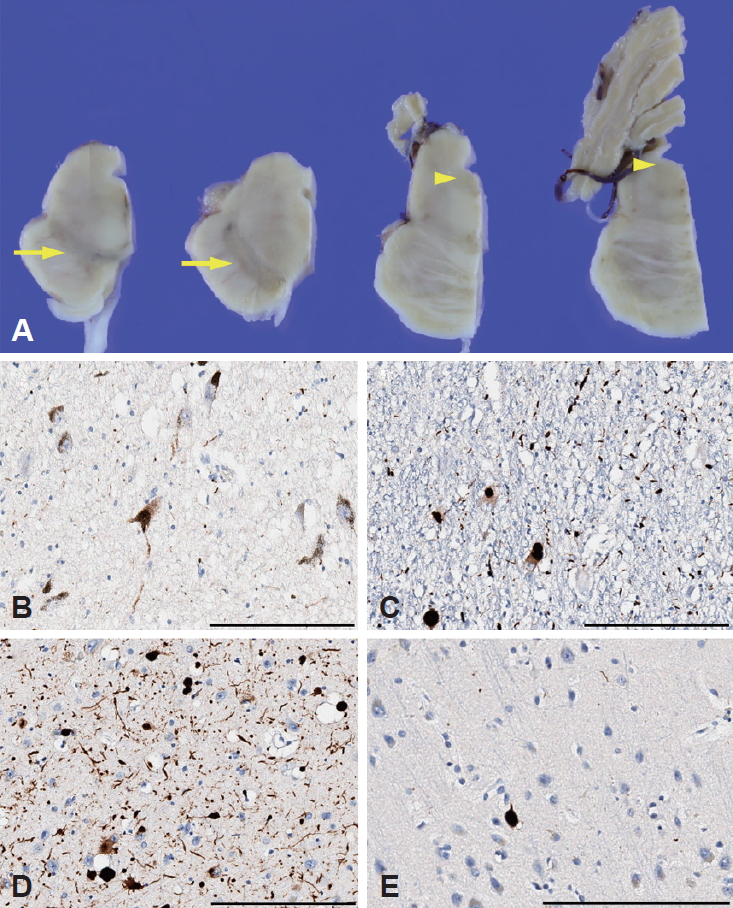
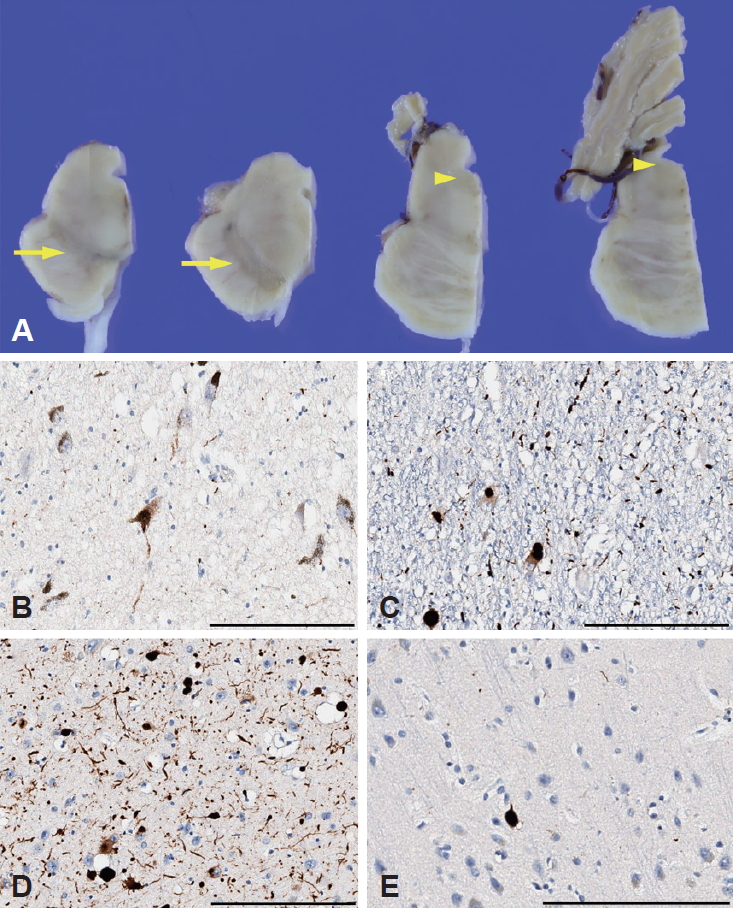
Postmortem neuropathological examination revealed a 1,210 g brain with mild diffuse brain atrophy. The SN and locus coeruleus (LC) showed severe loss of pigmentation (Figure 1A). Microscopic examination showed marked neuronal loss and extracellular neuromelanin pigments in the SN with severe Lewy-related pathology (Figure 1B). The LC and the trigeminal neurons of the pons also showed severe Lewy-related pathology (Figure 1C). Moderate Lewy-related pathology in the basal nucleus of Meynert and the amygdala (Figure 1D) and a few Lewy neurites in the olfactory bulb were found. However, in the neocortex, there was very mild Lewy-related pathology in the frontal cortex only (Figure 1E). Immunohistochemical staining for phosphorylated tau showed some tau-positive neurofibrillary tangles and neuropil threads in the entorhinal cortex, hippocampal CA1 regions and amygdala. Staining for amyloid-β and TAR DNA binding protein 43 (TDP-43) was negative in the brain. Postmortem genetic screening with 22 PD-associated genes (PARK1, PARK2, PARK5, PARK6, PARK7, PARK8, PARK9, PARK14, PARK15, PARK17, PARK18, GCH1, GBA, MAPT, etc.) by next-generation sequencing did not show any pathogenic variants.
The patient presented typical PD in which tremor-dominant parkinsonism could be managed with therapies initially, but in the advanced stage, motor and nonmotor complications were drug-refractory; however, she did not show overt dementia even after 18 years of disease duration. Although her cognition was assessed by brief tools and activities of daily living, it is possible she had subclinical mild cognitive impairment in the late-stage; however, her K-MMSE and FAB-K scores corresponded to normal cognition in PD and were above the mean values of cognitively normal elderly individuals, respectively, with adjusted age and education [5,6]. Concerning factors for disease progression in PD, the tremor-dominant phenotype tends to have fewer nonmotor symptoms and slower progression than non-tremor-dominant PD [1]. In terms if pathological features, limbic and cortical LB are the main driving cause of the progression of dementia in PD with or without additional Alzheimer’s pathology [3,4], although cortical LB does not always correlate with dementia in PD. Another contributor to cognitive decline in PD might be cholinergic deficits in the nucleus basalis of Meynert, although this deficit is likely to contribute to the fluctuations in alertness and attention in dementia with LBs [1]. However, prominent nonmotor symptoms, in this case, were pain and dream enactment behavior, both of which are related to the dysfunction of the brainstem, which modulates pain and rapid eye movement (REM) sleep [3]. Pathologically, Lewy-related pathology in the pons has shown associations with REM sleep behavior disorder, whereas the pathology in cutaneous nerves has been suggested as a contributor of pain in PD. In this clinico-pathological study, consistent with the observed motor and nonmotor features with preserved cognition, postmortem findings demonstrated brainstem-predominant Lewy-related pathology, and the only concomitant tau pathology restricted to the limbic areas was considered PART, which is commonly observed at autopsy among cognitively normal elderly individuals [7]. Further postmortem genetic studies failed to find genetic explanations for spared cognition, such as PARK2.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.14802/jmd.19059.
Supplementary Figure 1
Brain imaging. T2-weighted fluid-attenuated inversion recovery MRI of the brain at age 67 showed mild diffuse cerebral atrophy (A-D) with no significant atrophy in the medial temporal lobe (C and D).
jmd-19059-suppl.pdf
Notes
-
Conflicts of Interest
The authors have no financial conflicts of interest.
-
Author Contributions
Conceptualization: Beomseok Jeon. Funding acquisition: Sung-Hye Park and Beomseok Jeon. Investigation: All authors. Project administration: Beomseok Jeon. Resources: Sung-Hye Park, Sung Sup Park, and Beomseok Jeon. Supervision: Beomseok Jeon. Writing—original draft: Ji-Hyun Choi and Beomseok Jeon. Writing—review & editing: Ji-Hyun Choi and Beomseok Jeon.
Acknowledgments
- This research was supported by a fund (2018-ER6201-00) by Research of Korea Centers for Disease Control and Prevention. All samples derived from the National Biobank of Korea were obtained with informed consent under institutional review board-approved protocols.
Figure 1.Pathological features. Brain stem showed loss of pigmentation in the substantia nigra (arrows) and locus coeruleus (arrowheads) (A). α-synuclein immunohistochemistry staining showed severe neuronal loss and Lewy-related pathology (Lewy bodies and Lewy neurites) in the substantia nigra (B) and severe Lewy-related pathology in the locus coeruleus (C). There was moderate Lewy-related pathology in the basal nucleus of Meynert (D), while in the neocortex, only the frontal cortex showed very mild Lewy-related pathology (E). Scale bar = 200 μm.
REFERENCES
- 1. Kalia LV, Lang AE. Parkinson’s disease. Lancet 2015;386:896–912.ArticlePubMed
- 2. McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–1872.ArticlePubMed
- 3. Jellinger KA. Neuropathobiology of non-motor symptoms in Parkinson disease. J Neural Transm (Vienna) 2015;122:1429–1440.ArticlePubMedPDF
- 4. Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord 2008;23:837–844.ArticlePubMed
- 5. Kim JI, Sunwoo MK, Sohn YH, Lee PH, Hong JY. The MMSE and MoCA for screening cognitive impairment in less educated patients with Parkinson’s disease. J Mov Disord 2016;9:152–159.ArticlePubMedPMCPDF
- 6. Kim TH, Huh Y, Choe JY, Jeong JW, Park JH, Lee SB, et al. Korean version of frontal assessment battery: psychometric properties and normative data. Dement Geriatr Cogn Disord 2010;29:363–370.ArticlePubMed
- 7. Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 2014;128:755–766.ArticlePubMedPMCPDF
Citations
Citations to this article as recorded by

- Morphological differences between the two major subtypes of multiple system atrophy with cognitive impairment
Kurt A. Jellinger
Parkinsonism & Related Disorders.2023; 107: 105273. CrossRef - Neuropathological findings in multiple system atrophy with cognitive impairment
Kurt A. Jellinger
Journal of Neural Transmission.2020; 127(7): 1031. CrossRef
 , Sung-Hye Park3
, Sung-Hye Park3 , Sung Sup Park4
, Sung Sup Park4 , Beomseok Jeon1
, Beomseok Jeon1


 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite