Articles
- Page Path
- HOME > J Mov Disord > Volume 4(2); 2011 > Article
-
Original Article
Analysis of the Substantia Innominata Volume in Patients with Parkinson’s Disease with Dementia, Dementia with Lewy Bodies, and Alzheimer’s Disease - Hee Jin Kim, Ji Eun Lee, Soo Jeong Shin, Young Ho Sohn, Phil Hyu Lee
-
Journal of Movement Disorders 2011;4(2):68-72.
DOI: https://doi.org/10.14802/jmd.11014
Published online: October 30, 2011
Department of Neurology and Brain Research Institute, Yonsei University College of Medicine, Seoul, Korea
- Corresponding author: Phil Hyu Lee, MD, PhD, Department of Neurology, Yonsei University College of Medicine, 50 Yonsei-ro, Seodaemun-gu, Seoul 120-752, Korea, Tel +82-2-2228-1608, Fax +82-2-393-0705, E-mail phisland@chol.net
Copyright © 2011 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 13,551 Views
- 81 Download
- 12 Crossref
ABSTRACT
-
Background and Purpose
- The substantia innominata (SI) contains the nucleus basalis of Meynert, which is the major source of cholinergic input to the cerebral cortex. We hypothesized that degeneration of the SI and its relationship to general cognitive performance differs in amyloidopathy and synucleinopathy.
-
Methods
- We used magnetic resonance imaging (MRI)-based volumetric analysis to evaluate the SI volume in patients with amnestic mild cognitive impairment (aMCI), Alzheimer’s disease (AD), Parkinson’s disease-mild cognitive impairment (PD-MCI), PD with dementia (PDD), dementia with Lewy bodies (DLB), and healthy elderly controls. The correlation between SI volume and general cognitive performance, measured using the Korean version of the Mini-Mental State Examination (K-MMSE), was examined.
-
Results
- Compared to control subjects, the mean normalized SI volume was significantly decreased in all of the other groups. The normalized SI volume did not differ between the subjects with PDD and DLB, whereas it was significantly smaller in subjects with PDD (p = 0.029) and DLB (p = 0.011) compared with AD. In subjects with PD-related cognitive impairment (PD-MCI, PDD, or DLB), there was a significant positive correlation between the SI volume and K-MMSE score (r = 0.366, p < 0.001), whereas no correlation was seen in subjects with AD-related cognitive impairment (aMCI or AD).
-
Conclusions
- Our data suggest that the SI loss is greater in synucleinopathy-related dementia (PDD or DLB) than in AD and that the contribution of the SI to cognitive performance is greater in synucleinopathy than in amyloidopathy.
- Subjects
- We retrospectively enrolled 24 cognitively normal subjects, 45 patients with amnestic mild cognitive impairment (aMCI), 40 with AD, 38 with Parkinson’s disease-mild cognitive impairment (PD-MCI), 31 with PDD, and 22 with DLB from patients seen in the movement disorders and dementia outpatient clinic at a university hospital. Information on the patients’ cognitive deficits was obtained from a caregiver-based interview. To determine cognitive subsets in the diagnosis of MCI or dementia type, we used the Seoul Neuropsychological Screening Battery (SNSB).17 The SNSB covers attention, language, praxis, four elements of Gerstmann syndrome, visuoconstructive function, verbal and visual memory, and frontal/executive function. The quantifiable tests used were the digit span (forward and backward), the Korean version of the Boston Naming Test,18 the Rey Complex Figure Test (copying, immediate and 20-minute delayed recall, and recognition), the Seoul Verbal Learning Test (three learning-free recall trials of 12 words, 20-minute delayed recall trial for these 12 items, and a recognition test), the phonemic and semantic Controlled Oral Word Association Test, and the Stroop test (word and color reading of 112 items during a 2-minute period). Age-, sex-, and education-specific norms for each test based on 447 normal subjects are available.17 The scores of these quantifiable cognitive tests were classified as abnormal when they were below the sixteenth percentiles of the norms for the age-, sex-, and education-matched normal subjects.
- A patient was diagnosed with aMCI when at least one of the five cognitive domains, including the memory domain, was abnormal, and the patient did not meet the criteria for dementia. Using the concept of MCI suggested by Petersen et al.19 the diagnosis of MCI in patients with PD was made if at least one of the five cognitive domains was abnormal. All the aMCI and PD-MCI patients had scores on the Korean version of the MMSE (K-MMSE) above the sixteenth percentile for the age- and educational-appropriate norm and showed no evidence of deficits in activities of daily living.
- The clinical diagnosis of probable AD followed the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association guidelines.20 Patients were assigned to PDD when dementia occurred in the context of well-established PD (according to the UK Parkinson Disease Society Brain Bank criteria) with a time interval of at least 1 year after the onset of the motor syndrome.21,22 Probable DLB was diagnosed according to the consensus guidelines of the third report of the DLB Consortium.23
- Exclusion criteria were evidence of focal brain lesions, diffuse white matter hyperintensities, or multiple lacunae in the basal ganglia on MRI. Possible medical comorbidities were excluded by laboratory tests, including thyroid function tests, vitamin B12 and folic acid levels, and the VDRL test. Healthy age- and gender-matched elderly volunteers were used as controls for the MRI-based volumetric analysis. They were recruited by advertisements about the project or were healthy relatives of patients with movement disorders or dementia (n = 24, age = 72.0 years).
- MRI acquisition
- All scans of healthy controls and patients with AD, PDD, and DLB were acquired using a Philips 3.0-T scanner (Philips Intera; Philips Medical System, Best, The Netherlands) with a SENSE head coil (SENSE factor = 2). Head motion was minimized using restraining foam pads provided by the manufacturer. A high-resolution T1-weighted MRI volume dataset was obtained for all subjects using the 3D T1-TFE sequence configured with the following acquisition parameters: axial acquisition with a 224 × 256 matrix; 256 × 256 reconstructed matrix with 182 slices in the coronal plane; 1-mm-thick sections; 220-mm field of view; 0.98 × 0.98 × 1.2 mm3 voxels; TE, 4.6 ms; TR, 9.6 ms; flip angle, 8°; slice gap, 0 mm.
- Volumetric determination of SI
- The delineation of the SI on MRI was based on the method reported by George et al.1 The SI volume was measured on three consecutive coronal sections using the following demarcation. For the first section at the level of the crossing of the anterior commissure, the ventral aspect of the globus pallidus demarcated the dorsal border of the SI, and the ventral border was the base of the brain containing the anterior perforated space. The medial border of the SI was defined by a vertical line extending from the ventrolateral border of the bed nucleus of the brain. The lateral border extended to the medial aspect of the putamen. The second section was between the first and third sections, and the third section was at the level of the emergence of the anterior commissure from the temporal lobe. To correct for individual brain size, the volumes were normalized by dividing by the total intracranial volume derived from sagittally formatted 10-mm slices. The normalized SI volume was defined by the following formula: total SI volume (mm3)/total intracranial volume (mm3) × 10,000. The intra- and inter-rater reliability were 0.82 and 0.80, respectively.
- Statistical analysis
- Data are expressed as the mean ± standard deviation. One-way analysis of variance followed by post hoc comparisons was used to compare group differences in age, duration of education, total intracranial volume, normalized SI volume, and the K-MMSE and Clinical Dementia Rating scores. The chi-square test was used to compare the gender distribution. Pearson’s correlation analysis was used to analyze the relationship between SI volume and the K-MMSE score. Statistical analysis was performed using SPSS ver. 17.0, and p-values < 0.05 were considered significant.
Methods
- Demographic characteristics
- The demographic characteristics of the patients are shown in Table 1. No significant difference in age, gender, education level, or total intracranial volume was found among the control, aMCI, PD-MCI, AD, PDD, and DLB groups.
- Comparison of SI volume among groups
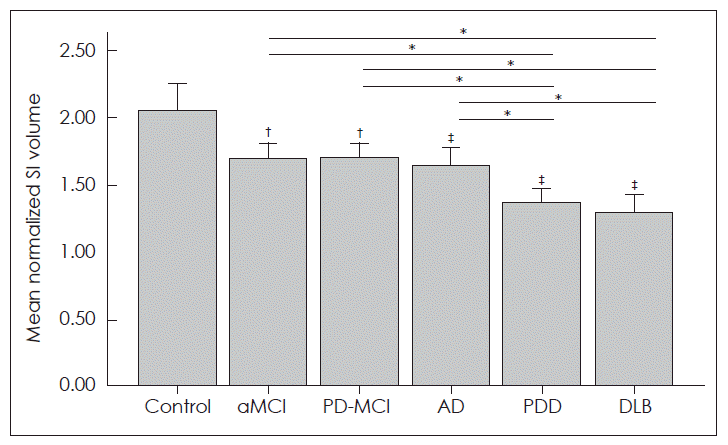
- Compared with the control subjects (2.05 ± 0.52), the mean normalized SI volume was significantly smaller in the patients with aMCI (1.70 ± 0.39, p = 0.006), PD-MCI (1.70 ± 0.35, p = 0.008), AD (1.65 ± 0.41, p < 0.001), PDD (1.36 ± 0.32, p < 0.001), and DLB (1.30 ± 0.33, p < 0.001). Compared with the patients with aMCI, the normalized SI volume was significantly smaller in the patients with PDD (p = 0.003) and DLB (p = 0.001); however, the volume did not differ significantly from those with AD. Compared with the patients with PD-MCI, the normalized SI volume was significantly smaller in the patients with PDD (p = 0.003) and DLB (p = 0.001). On comparison among dementia subjects, the normalized SI volume did not differ between patients with PDD and DLB. However, the normalized SI volume was significantly decreased in subjects with PDD (p = 0.029) and DLB (p = 0.011) compared with AD subjects (Figure 1).
- Correlation analysis of SI volume and general cognition
- In patients with AD-related cognitive impairment (aMCI or AD), there was no significant correlation between the SI volume and general cognitive function, as measured using the K-MMSE score (r = 0.045, p = 0.685) (Figure 2B). In contrast, the SI volume in subjects with PD-related cognitive impairment (PD-MCI, PDD, or DLB) showed a significant positive correlation with general cognitive function (r = 0.366, p < 0.001) (Figure 2A).
Results
- This study evaluated whether degeneration of the SI and its relationship to general cognitive performance differ in AD- and PD-related cognitive impairments. The major findings of this study were 1) the mean normalized SI volume was significantly smaller in patients with either aMCI or PD-MCI, 2) the loss of SI volume was greater in PD-related dementia (PDD or DLB) than in AD, and 3) SI volume was significantly correlated with general cognitive decline only in patients with PDD or DLB.
- A few studies have examined SI volume using MRI in patients with MCI; however, the results are conflicting. Muth et al.24 and Grothe et al.25 reported that the SI volume was significantly decreased in MCI compared with normal elderly controls, whereas George et al.1 showed that the SI volume in MCI did not differ from that in controls. In patients with PD, we previously reported that the SI volume was significantly decreased in PD-MCI compared with the controls.26 In the present study, we found that the SI volume was significantly decreased in aMCI and PD-MCI, suggesting that cholinergic degeneration occurs with MCI status regardless of the AD or PD prototypes.
- Studies that compared the SI atrophy in AD and DLB by measuring the SI length12,13 have shown that the thickness of the SI was significantly greater in AD than in DLB, which concurs with our SI volume analysis. However, no previous reports have compared the SI atrophy between PDD and AD. Our data showed that the normalized SI volume was significantly smaller not only in subjects with DLB but also in subjects with PDD compared with AD. Because a study showed that cortical cholinergic activity is more severely affected in parkinsonian dementia than in Alzheimer disease,14 our result suggests that the degeneration of the cholinergic system arising from the nucleus basalis of Meynert is more closely coupled with PD-related dementia than with AD-related dementia. On comparing subjects with alpha-synuclein-related dementia, the normalized SI volume did not differ between patients with PDD and DLB. This result is in line with a functional PET imaging study that showed that the cholinergic activity did not differ between PDD and DLB.8 Accordingly, previous data and ours support the concept that PDD and DLB lie on a common disease spectrum with respect to cholinergic pathophysiology.
- Contrary to previous reports,1 in the subjects with AD-related cognitive impairment (aMCI or AD), there was no significant correlation between the SI volume and general cognitive function. In contrast, there was a significant positive correlation between the SI volume and general cognitive function in the subjects with PD-related cognitive impairment (PD-MCI, PDD, or DLB). This suggests that cognitive performance is closely dependent on the cholinergic system in PD-related cognitive impairment, whereas this relationship is weak in AD-related cognitive impairment. Furthermore, this finding in our study is supported by a previous clinical study indicating that the response to cholinesterase inhibitors is greater in DLB than in AD.27–29
- In summary, our data suggest that the SI volume loss is greater in alpha-synucleinopathy-related dementia (PDD or DLB) than in AD, and the contribution of the SI to cognitive performance is greater in alpha-synucleinopathy-related cognitive impairments than in AD.
Discussion


aMCI: amnestic mild cognitive impairment, PD-MCI: Parkinson’s disease-mild cognitive impairment, AD: Alzheimer’s disease, PDD: Parkinson’s disease with dementia, DLB: dementia with Lewy bodies, SI: substantia innominata, CDR: Clinical Dementia Rating, K-MMSE: Korean version of the Mini-Mental State Examination, NS: not significant.
- 1. George S, Mufson EJ, Leurgans S, Shah RC, Ferrari C, deToledo-Morrell L. MRI-based volumetric measurement of the substantia innominata in amnestic MCI and mild AD. Neurobiol Aging 2011;32:1756–1764.ArticlePubMedPMC
- 2. Oikawa H, Sasaki M, Ehara S, Abe T. Substantia innominata: MR findings in Parkinson’s disease. Neuroradiology 2004;46:817–821.ArticlePubMed
- 3. Sassin I, Schultz C, Thal DR, Rüb U, Arai K, Braak E, et al. Evolution of Alzheimer’s disease-related cytoskeletal changes in the basal nucleus of Meynert. Acta Neuropathol 2000;100:259–269.ArticlePubMed
- 4. Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR. Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol 1981;10:122–126.ArticlePubMed
- 5. Kuhl DE, Koeppe RA, Minoshima S, Snyder SE, Ficaro EP, Foster NL, et al. In vivo mapping of cerebral acetylcholinesterase activity in aging and Alzheimer’s disease. Neurology 1999;52:691–699.ArticlePubMed
- 6. Lippa CF, Smith TW, Perry E. Dementia with Lewy bodies: choline acetyltransferase parallels nucleus basalis pathology. J Neural Transm 1999;106:525–535.ArticlePubMed
- 7. Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003;24:197–211.ArticlePubMed
- 8. Klein JC, Eggers C, Kalbe E, Weisenbach S, Hohmann C, Vollmar S, et al. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology 2010;74:885–892.ArticlePubMed
- 9. Shimada H, Hirano S, Shinotoh H, Aotsuka A, Sato K, Tanaka N, et al. Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology 2009;73:273–278.ArticlePubMed
- 10. Förstl H, Burns A, Luthert P, Cairns N, Levy R. The Lewy-body variant of Alzheimer’s disease. Clinical and pathological findings. Br J Psychiatry 1993;162:385–392.ArticlePubMed
- 11. Hansen L, Salmon D, Galasko D, Masliah E, Katzman R, DeTeresa R, et al. The Lewy body variant of Alzheimer’s disease: a clinical and pathologic entity. Neurology 1990;40:1–8.Article
- 12. Hanyu H, Tanaka Y, Shimizu S, Sakurai H, Iwamoto T, Abe K. Differences in MR features of the substantia innominata between dementia with Lewy bodies and Alzheimer’s disease. J Neurol 2005;252:482–484.ArticlePubMed
- 13. Hanyu H, Shimizu S, Tanaka Y, Hirao K, Iwamoto T, Abe K. MR features of the substantia innominata and therapeutic implications in dementias. Neurobiol Aging 2007;28:548–554.ArticlePubMed
- 14. Bohnen NI, Kaufer DI, Ivanco LS, Lopresti B, Koeppe RA, Davis JG, et al. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch Neurol 2003;60:1745–1748.ArticlePubMed
- 15. Hanyu H, Tanaka Y, Sakurai H, Takasaki M, Abe K. Atrophy of the substantia innominata on magnetic resonance imaging and response to donepezil treatment in Alzheimer’s disease. Neurosci Lett 2002;319:33–36.ArticlePubMed
- 16. Hanyu H, Asano T, Sakurai H, Tanaka Y, Takasaki M, Abe K. MR analysis of the substantia innominata in normal aging, Alzheimer disease, and other types of dementia. AJNR Am J Neuroradiol 2002;23:27–32.PubMedPMC
- 17. Kang Y, Na DL. Seoul Neuropsychological Screening Battery. Incheon: Human Brain Research & Consulting Co.; 2003.
- 18. Kim H, Na DL. Normative data on the Korean version of the Boston Naming Test. J Clin Exp Neuropsychol 1999;21:127–133.ArticlePubMed
- 19. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–308.ArticlePubMed
- 20. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939–944.ArticlePubMed
- 21. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184.ArticlePubMedPMC
- 22. Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 2007;22:1689–1707.quiz 1837. ArticlePubMed
- 23. McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–1872.ArticlePubMed
- 24. Muth K, Schönmeyer R, Matura S, Haenschel C, Schröder J, Pantel J. Mild cognitive impairment in the elderly is associated with volume loss of the cholinergic basal forebrain region. Biol Psychiatry 2010;67:588–591.ArticlePubMed
- 25. Grothe M, Zaborszky L, Atienza M, Gil-Neciga E, Rodriguez-Romero R, Teipel SJ, et al. Reduction of basal forebrain cholinergic system parallels cognitive impairment in patients at high risk of developing Alzheimer’s disease. Cereb Cortex 2010;20:1685–1695.ArticlePubMedPMC
- 26. Choi SH, Jung TM, Lee JE, Lee SK, Sohn YH, Lee PH. Volumetric analysis of the substantia innominata in patients with Parkinson’s disease according to cognitive status. Neurobiol Aging 2011;[Epub ahead of print]. Article
- 27. Samuel W, Caligiuri M, Galasko D, Lacro J, Marini M, McClure FS, et al. Better cognitive and psychopathologic response to donepezil in patients prospectively diagnosed as dementia with Lewy bodies: a preliminary study. Int J Geriatr Psychiatry 2000;15:794–802.ArticlePubMed
- 28. Levy R, Eagger S, Griffiths M, Perry E, Honavar M, Dean A, et al. Lewy bodies and response to tacrine in Alzheimer’s disease. Lancet 1994;343:176.Article
- 29. Simard M, van Reekum R. The acetylcholinesterase inhibitors for treatment of cognitive and behavioral symptoms in dementia with Lewy bodies. J Neuropsychiatry Clin Neurosci 2004;16:409–425.ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Deep Learning Segmentation of the Nucleus Basalis of Meynert on 3T MRI
D.J. Doss, G.W. Johnson, S. Narasimhan, J.S. Shless, J.W. Jiang, H.F.J. González, D.L. Paulo, A. Lucas, K.A. Davis, C. Chang, V.L. Morgan, C. Constantinidis, B.M. Dawant, D.J. Englot
American Journal of Neuroradiology.2023; 44(9): 1020. CrossRef - Manual and automated analysis of atrophy patterns in dementia with Lewy bodies on MRI
Eya Khadhraoui, Sebastian Johannes Müller, Niels Hansen, Christian Heiner Riedel, Philip Langer, Charles Timäeus, Jens Wiltfang, Caroline Bouter, Claudia Lange, Marielle Ernst
BMC Neurology.2022;[Epub] CrossRef - Cholinergic white matter pathways in dementia with Lewy bodies and Alzheimer’s disease
Julia Schumacher, Nicola J Ray, Calum A Hamilton, Paul C Donaghy, Michael Firbank, Gemma Roberts, Louise Allan, Rory Durcan, Nicola Barnett, John T O’Brien, John-Paul Taylor, Alan J Thomas
Brain.2022; 145(5): 1773. CrossRef - Metric magnetic resonance imaging analysis reveals pronounced substantia-innominata atrophy in dementia with Lewy bodies with a psychiatric onset
Niels Hansen, Sebastian Johannes Müller, Eya Khadhraoui, Christian Heiner Riedel, Philip Langer, Jens Wiltfang, Charles-Arnold Timäus, Caroline Bouter, Marielle Ernst, Claudia Lange
Frontiers in Aging Neuroscience.2022;[Epub] CrossRef - In vivo nucleus basalis of Meynert degeneration in mild cognitive impairment with Lewy bodies
Julia Schumacher, John-Paul Taylor, Calum A. Hamilton, Michael Firbank, Ruth A. Cromarty, Paul C. Donaghy, Gemma Roberts, Louise Allan, Jim Lloyd, Rory Durcan, Nicola Barnett, John T. O'Brien, Alan J. Thomas
NeuroImage: Clinical.2021; 30: 102604. CrossRef - EEG alpha reactivity and cholinergic system integrity in Lewy body dementia and Alzheimer’s disease
Julia Schumacher, Alan J. Thomas, Luis R. Peraza, Michael Firbank, Ruth Cromarty, Calum A. Hamilton, Paul C. Donaghy, John T. O’Brien, John-Paul Taylor
Alzheimer's Research & Therapy.2020;[Epub] CrossRef - Transcriptional network analysis in frontal cortex in Lewy body diseases with focus on dementia with Lewy bodies
Gabriel Santpere, Paula Garcia‐Esparcia, Pol Andres‐Benito, Belen Lorente‐Galdos, Arcadi Navarro, Isidro Ferrer
Brain Pathology.2018; 28(3): 315. CrossRef - Nucleus Basalis of Meynert Stimulation for Dementia: Theoretical and Technical Considerations
Deepak Kumbhare, Viktoras Palys, Jamie Toms, Chathurika S. Wickramasinghe, Kasun Amarasinghe, Milos Manic, Evan Hughes, Kathryn L. Holloway
Frontiers in Neuroscience.2018;[Epub] CrossRef - Dementia with Lewy Bodies: Molecular Pathology in the Frontal Cortex in Typical and Rapidly Progressive Forms
Paula Garcia-Esparcia, Irene López-González, Oriol Grau-Rivera, María Francisca García-Garrido, Anusha Konetti, Franc Llorens, Saima Zafar, Margarita Carmona, José Antonio del Rio, Inga Zerr, Ellen Gelpi, Isidro Ferrer
Frontiers in Neurology.2017;[Epub] CrossRef - Meynert’s Nucleus Complex White Matter Abnormalities in Autism Spectrum Disorders: An MRI Study
Matteo Pardini, Francesco G. Garaci, Laszlo Zaborszky, Filadelfo Coniglione, Gianluca Serafini, Martina Siracusano, Francesca Benassi, Leonardo Emberti Gialloreti
Journal of Intellectual Disability - Diagnosis and Treatment.2017; 4(4): 185. CrossRef - Biomarkers for dementia and mild cognitive impairment in Parkinson's disease
Manuel Delgado‐Alvarado, Belén Gago, Irene Navalpotro‐Gomez, Haritz Jiménez‐Urbieta, María C. Rodriguez‐Oroz
Movement Disorders.2016; 31(6): 861. CrossRef - Nucleus basalis of Meynert revisited: anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease
Alan King Lun Liu, Raymond Chuen-Chung Chang, Ronald K. B. Pearce, Steve M. Gentleman
Acta Neuropathologica.2015; 129(4): 527. CrossRef
Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite