Association between Olfactory Deficit and Motor and Cognitive Function in Parkinson’s Disease
Article information
Abstract
Objective
To investigate whether baseline olfactory dysfunction in Parkinson’s disease (PD) patients is associated with baseline and longitudinal motor and cognitive function.
Methods
We recruited 228 drug-naïve PD patients who were followed for a mean of 6 years. Patients underwent the Cross-Cultural Smell Identification Test (CCSIT), a neuropsychological test, and N-(3-[18F]fluoropropyl)-2β-carbomethoxy-3β-(4-iodophenyl) nortropane positron emission tomography within 6 months of the baseline evaluation. Olfactory dysfunction was categorized as normosmia (CCSIT score ≥ 9), hyposmia (CCSIT score 5–8), and anosmia (CCSIT score ≤ 4). During the follow-up period, we investigated changes in the levodopa-equivalent dose (LED) and the occurrence of wearing-off, levodopa-induced dyskinesia, and dementia.
Results
Among the PD patients, 80.7% were hyposmic at the time of diagnosis, and 26.1% were anosmic. Baseline olfactory dysfunction was not associated with either initial parkinsonian motor symptoms or with the longitudinal LED increment and motor complications. Meanwhile, the anosmic group had lower baseline scores on the Korea version of the Boston Naming Test and Stroop color reading test than the normosmic and hyposmic groups. The anosmic group exhibited a higher rate of conversion to dementia than the normosmic [adjusted hazard ratio (HR) 3.99, 95% confidence interval (CI) 1.08–14.72] and hyposmic (adjusted HR 2.48, 95% CI 1.15–5.32) PD groups, regardless of baseline motor deficits and cognitive status.
Conclusion
Baseline olfactory dysfunction was not associated with motor deficits and complications, but it was associated with cognitive dysfunction and prognosis, suggesting that severe olfactory impairment may reflect early cortical involvement, probably in the frontotemporal region, and rapid spreading of Lewy body pathology.
Olfactory dysfunction is an important prodromal non-motor symptom of Parkinson’s disease (PD) [1]. It often predates the diagnosis of PD by at least four years [2], and 70–80% of patients with PD already have decreased olfaction at the time of diagnosis [3]. Braak et al. [4] found that the anterior olfactory nucleus is one of the earliest sites where Lewy bodies are observed. Moreover, olfactory deficits significantly increase the rate of phenoconversion from rapid eye movement sleep behavior disorder (RBD) to PD [5]. Accordingly, olfactory dysfunction has been a potential biomarker for the early and differential diagnosis of PD.
Many studies have investigated the association between olfactory impairment and various motor and non-motor symptoms. It is still unclear whether olfactory dysfunction is associated with parkinsonian motor deficits. The Parkinson’s Progression Markers Initiative, a study that recruited PD patients with a disease duration of less than two years, reported that olfactory impairment was not associated with the Unified Parkinson’s Disease Rating Scale (UPDRS) motor score, disease duration, or dopamine transporter (DAT) deficit [6]. Meanwhile, a pathologic study revealed that synucleinopathy density scores in the olfactory bulb are correlated with UPDRS motor scores. The olfactory function score was correlated with the UPDRS motor score and putaminal DAT binding [7]. Regarding cognition, olfactory dysfunction has been consistently reported to be associated with cognitive impairment and conversion to dementia in PD [3,6,8-10]. In a positron emission tomography (PET) study, olfactory identification impairment was correlated with DAT activity in the caudate nuclei [11].
In this study, we investigated the association of olfactory dysfunction with baseline motor and cognitive dysfunction, DAT activity, levodopa-equivalent dose (LED) increments, and the occurrence of motor and cognitive complications in drug-naïve PD patients during a follow-up period of 3–10 years. We hypothesized that olfactory dysfunction may be differentially associated with motor and cognitive deficits and their prognoses.
MATERIALS & METHODS
Study participants
In this retrospective cohort study, we consecutively recruited 228 drug-naïve patients with PD who visited the movement disorders outpatient clinic at Severance Hospital, Yonsei University Health System, from June 2009 to March 2016; all included patients has been followed for at least three years and up to 10 years. All subjects had undergone neurological examination, UPDRS, Cross-Cultural Smell Identification Test (CCSIT), neuropsychological test, brain magnetic resonance imaging (MRI), and N-(3-[18F]fluoropropyl)-2β-carbomethoxy-3β-(4-iodophenyl) nortropane (18F-FP-CIT) PET within six months. The diagnosis of PD was based on the clinical diagnostic criteria of the United Kingdom PD Society Brain Bank. All patients with PD showed decreased DAT uptake in the posterior putamen and responded to dopaminergic medication. RBD was assessed with the RBD Screening Questionnaire. The LED was calculated according to a previously described method [12]. We excluded patients with severe white matter hyperintensities; multiple lacunes in the basal ganglia or hydrocephalus on MRI; other neurologic, psychiatric, or metabolic illnesses; dementia at baseline evaluation; atypical parkinsonism such as multiple system atrophy, corticobasal degeneration, or progressive supranuclear palsy; or any conditions affecting olfactory function, including history of nasal surgery, chronic sinonasal disease, recent upper respiratory tract infections, or major head trauma.
This study was approved by the Institutional Review Board of Yonsei University Severance Hospital (IRB No. 4-2014-0637). Given the retrospective nature of the present study, written informed consent was waived.
Assessment of olfactory function and grouping according to olfactory function
Olfactory function was assessed with the CCSIT, which consists of 12 odors [13]. Participants were required to scratch the panel, sniff the sample, and select from one of four possible answers. Their scores were calculated as the sum of correct responses. Based on previous studies, normosmia was diagnosed when the score ranged from 9 to 12, hyposmia was diagnosed when the score ranged from 5 to 8, and anosmia was diagnosed when the score ranged from 0 to 4 [14].
PET image acquisition
18F-FP-CIT PET scans were obtained using a Discovery 600 system (General Electric Healthcare, Milwaukee, MI, USA). A dose of 185 MBq (5 mCi) of 18F-FP-CIT was injected intravenously during the procedure. Ninety minutes after the injection, images were acquired during a 20-minute session, followed by a CT scan for attenuation correction. The spiral CT scan was performed with a 0.8 s/rotation at 120 kVp, 10 mA, 3.75 mm slice thickness, 0.625 mm collimation and 9.375 mm table feed per rotation. Images were reconstructed using the ordered subset expectation maximization algorithm with ƒ iterations and 32 subsets. A Gaussian filter with a full-width at half-maximum of 4 mm was applied to each reconstructed PET image, which was made up of a 256 × 256 matrix with 0.98 mm pixel and 0.98 mm slice thickness.
Quantitation of the 18F FP-CIT PET-CT images
We analyzed the 18F-FP-CIT PET images according to the method described in a previous study [15]. Image processing was performed using Statistical Parametric Mapping 8 (Wellcome Department of Imaging Neuroscience, Institute of Neurology, University College London, London, UK) with MATLAB R2013a for Windows (Math Works, Natick, MA, USA). Quantitative analyses were based on the volumes of interest (VOIs), which were defined based on a template in standard space. All reconstructed PET images were spatially normalized to Talairach space by using a standard 18F-FP-CIT PET template, which was made inhouse, as described previously [16]. Four VOIs of the bilateral caudate nuclei and putamen and one occipital VOI were drawn on a coregistered spatially normalized single T1 MRI and 18F-FPCIT PET template image on MRIcro version 1.37 (Chris Rorden, Columbia, SC, USA). These VOIs were adjusted by minor translation using our in-house editing software, ANIQUE (Department of Nuclear Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea). The level of DAT activity in each VOI was calculated in terms of the specific to non-specific binding ratio as follows: (mean standardized uptake of the striatal subregional VOI-mean standardized uptake of the occipital VOI)/mean standardized uptake of the occipital VOI. For non-specific binding, the mean standardized uptake value was calculated by drawing two occipital VOIs, one on each side.
Clinical parameters and assessment of motor function
At baseline, parkinsonian motor symptoms were assessed during a drug-naïve state at the time of 18F-FP-CIT PET acquisition using the UPDRS motor (UPDRS-III) subscales. Tremor and non-tremor scores were calculated in each patient according to a previously described method, and patients were classified into three clinical subtypes: tremor-dominant, akinetic-rigid, and mixed [17]. DAT activity in the putamen was used to evaluate striatal function in relation to parkinsonian motor function.
We investigated longitudinal changes in the dose of dopaminergic medication using the LED during the first three years of follow-up, which can indirectly reflect the progression of parkinsonian motor symptoms. We also assessed the occurrence of motor complications, including wearing-off (WO) and levodopainduced dyskinesia (LID), during the follow-up period. Patients with PD visited the outpatient clinic every three to six months, and two movement disorders experts (Y.H.S. and P.H.L.) carefully examined them for the presence of motor complications based on the patient history provided by the patients and their caregivers or by performing direct neurological examination at every visit. We considered the date on which the PD patients or their caregivers reported the occurrence of motor complications or the date on which motor complications were first observed in the clinic as the date of the occurrence of motor complications.
Clinical parameters and assessment of cognitive function
At baseline, all participants underwent a standardized neuropsychological battery called the Seoul Neuropsychological Screening Battery [18], which is composed of the following scorable tests: digit span (forward and backward), repetition, the Korean version of the Boston Naming Test (K-BNT), the 6-point pentagon drawing test, the Rey-Osterrieth Complex Figure Test (copying, immediate and 20-min delayed recall, and recognition), the Seoul Verbal Learning Test (SVLT; immediate recall, 20-min delayed recall, and recognition), contrasting programming and the go/no-go test, the clock drawing test, the phonemic and semantic Controlled Oral Word Association Test, the Stroop Test (word and color reading), the Korean version of the Mini-Mental State Examination (K-MMSE), and the Clinical Dementia Rating. Each score was converted into a standardized score (z-score) based on age- and education-specific norms. Patients were diagnosed with PD with intact cognition when impairments were observed in less than two items on the detailed neuropsychological tests. In accordance with the Movement Disorder Society Task Force guidelines, a diagnosis of PD with mild cognitive impairment (MCI) was made when there was no evidence of abnormal activities in daily living (ADLs). DAT activity in the caudate was used to assess striatal function in relation to cognitive function.
PD patients were followed-up for more than three years, and they performed neuropsychological tests or the K-MMSE every two to three years or when they or their caregivers complained of cognitive decline in their daily living activities. Patients were diagnosed with PD with dementia if they fulfilled the clinical criteria for probable PD with dementia based on the Movement Disorder Society Task Force guideline (both level I and II testing) with evidence of abnormal ADLs. Impairment of cognitive decline in ADLs was assessed both clinically and with the ADL scale.
Statistical analyses
The baseline demographic characteristics of the study participants were analyzed using the independent t-test or Pearson χ2-test as appropriate. To compare baseline motor and cognitive parameters, we performed analysis of covariance (ANCOVA) using age, sex, and the interval from PD onset to CCSIT evaluation as covariates for continuous variables, and we performed the Pearson χ2-test for categorical variables. Partial correlation analysis was performed to investigate the correlation between the CCSIT score and neuropsychological performance. Corrections for multiple analyses across 15 neuropsychological items were performed using the false discovery rate (FDR) method in the ANCOVA and partial correlation analyses. A linear mixed model was used to examine the differences in the rates of longitudinal changes in the LED among the groups after controlling for the same covariates. The effects of the PD subgroups on changes in the LED over time were tested using the time by PD subgroup interaction term. To investigate the hazard ratio (HR) of developing WO, LID, or conversion to dementia, we used a Cox proportional hazards model that included the same covariates. We further adjusted for the total UPDRS-III score and cognitive status in the Cox proportional hazards model for conversion to dementia according to olfactory dysfunction. Data were analyzed using SPSS software, ver. 23 (IBM Corp., Armonk, NY, USA). p-values less than 0.05 were considered significant.
RESULTS
Demographic and clinical characteristics of the study participants
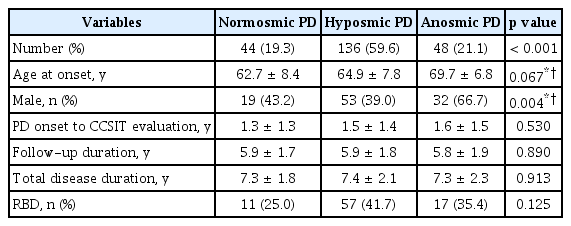
According to the baseline CCSIT scores, 19.3% of PD patients had normal olfactory function, 59.6% had mildly decreased olfaction, and 21.1% had severe olfactory loss at the time of PD diagnosis (Table 1). Anosmic PD patients were older at PD onset, and a higher proportion of these patients were male compared to the normosmic and hyposmic PD groups. The interval from PD onset to CCSIT evaluation, follow-up duration, total disease duration, and proportion of RBD were not different among the groups.
Baseline motor profiles of the study participants
The baseline parkinsonian motor score and DAT activity in the putamen were not different among the PD groups after controlling for age, sex, and the interval from PD onset to CCSIT evaluation (Table 2). There was also no difference in the proportion of clinical subtypes among the PD groups.
Baseline cognitive profiles of the study participants
At the baseline neuropsychological evaluation, the anosmic PD group had a higher proportion of MCI than the normosmic PD group (Table 2). There was no difference in the proportion of patients with amnestic MCI among the PD groups. Among the neuropsychological battery of tests, the anosmic PD patients showed significantly poorer performance on the K-BNT (p = 0.005) and Stroop color reading (p = 0.023) items when uncorrected. Additionally, we investigated the association between olfactory function and neuropsychological performance using the CCSIT as a continuous variable. The CCSIT score was significantly correlated with the K-BNT, Stroop color reading, and SVLT recognition scores after applying the FDR method (Supplementary Table 1 in the online-only Data Supplement). The three PD groups showed no differences in any of the other neuropsychological tests or in the DAT activity in the caudate.
Longitudinal changes in the LED and occurrence of motor complications
A linear mixed model analysis showed that there were no differences in the rate of LED changes during the first three years of follow-up among the PD groups (time by PD subgroup interaction, p = 0.144) (Figure 1). A Cox proportional hazard model according to olfactory dysfunction in PD patients showed no difference in the occurrence of WO (Figure 2A) and LID (Figure 2B) among the PD groups.
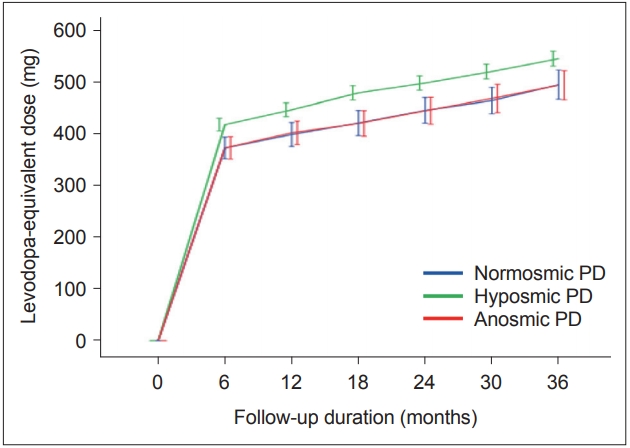
Longitudinal changes in the levodopa-equivalent dose with time among the Parkinson’s disease (PD) groups according to olfactory function. There was no significant interaction between PD groups and time in the linear mixed model (p = 0.144).
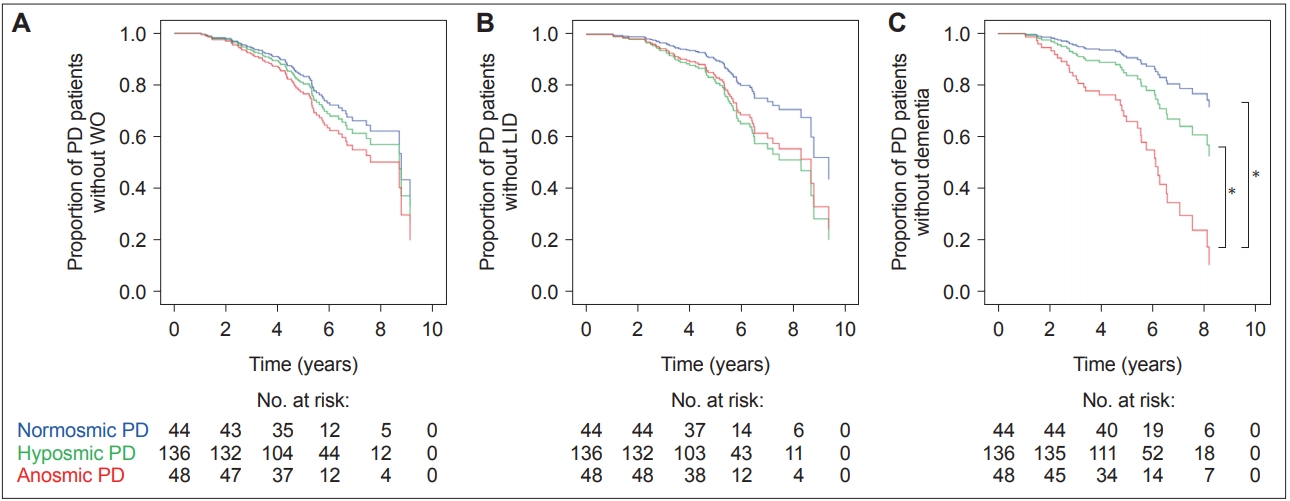
Estimated survival function for the Cox regression of time to (A) the onset of WO, (B) the onset of LID, and (C) the conversion to dementia among the PD groups according to olfactory function. *a significant difference between the PD groups. LID: levodopa-induced dyskinesia, PD: Parkinson’s disease, WO: wearing-off.
Occurrence of PD dementia
The anosmic PD group showed a significantly higher conversion rate to dementia than the normosmic [HR 3.89, 95% confidence interval (CI) 1.06–14.21] and hyposmic (HR 2.20, 95% CI 1.04–4.66) PD groups, independent of age, sex, and interval from PD onset to CCSIT evaluation (Figure 2C, Table 3). When we further adjusted for the total UPDRS-III score and cognitive status, the anosmic group still had a higher conversion rate to dementia than the normosmic (HR 3.99, 95% CI 1.08–14.72) and hyposmic (HR 2.48, CI 1.15–5.32) PD groups. Additionally, we investigated the HR of olfactory dysfunction for dementia conversion using the CCSIT as a continuous variable. The HR of the baseline CCSIT score for the risk of future development of dementia was 0.73 (95% CI 0.61–0.87) after controlling for age, sex, interval from PD onset to CCSIT evaluation, total UPDRS-III score, and cognitive status.
DISCUSSION
The present study investigated the association of baseline olfactory function with motor and cognitive deficits and the longitudinal motor and cognitive outcomes in PD. The major findings were as follows: 1) 81.1% of PD patients had decreased olfaction at the time of diagnosis, and 26.1% of PD patients had severe loss of olfaction; 2) olfactory dysfunction was not associated with initial parkinsonian motor symptoms but was associated with baseline dysfunction in the K-BNT and Stroop color reading test, which reflect deficits in frontal and temporal functions; 3) baseline olfactory dysfunction was neither associated with motor progression nor with the occurrence of motor complications; and 4) baseline olfactory dysfunction predicted the progression to dementia regardless of baseline motor deficits and cognitive status. Taken together, these results suggest that, while baseline olfactory dysfunction is not associated with motor deficits and complications, it is associated with cognitive dysfunction and prognosis.
Severely decreased olfaction at the time of PD diagnosis was associated with MCI and poor performance in the K-BNT and Stroop color reading test. Previous studies reported that PD patients with MCI have worse olfactory function than those with intact cognition [9,19,20]. This suggests the early involvement of the cortical Lewy body, a pathological hallmark of PD, in cases where there is initiation of neuropathological spread of the neurodegenerative process through an olfactory route [21]. However, heterogeneous results have been reported regarding which specific cognitive domain is impaired in association with olfactory dysfunction in PD: attentional deficit [10], visuospatial dysfunction [19], memory impairment [22-24], or executive dysfunction [22,24]. This can be attributed to the different ways of evaluating olfactory and cognitive functions and to studies that enrolled PD patients with various disease stages. Since antiparkinsonian drugs have the potential to influence a range of cognitive functions, it is necessary to investigate the association between olfactory and cognitive functions in early and drug-naïve PD patients. Furthermore, as we enrolled PD patients with a mean disease duration of less than 2 years at baseline evaluation, we can infer that, from the early stage of PD, olfactory deficits are closely related to specific cognitive dysfunction, and they are predictive of future development of dementia. Imaging studies have revealed that olfactory dysfunction is associated with olfaction-related cortical atrophy, including piriform and orbitofrontal cortices [25] or white matter disintegrity near the orbitofrontal cortex in PD [20]. Since olfactionrelated structures are located near and within the basal and medial frontal regions and the inferior and medial temporal areas, Lewy body entry through the nasal route could primarily affect these regions from the early stage of PD [21]. The suggested neural correlate involved in naming is the inferior temporal cortex [26] and that of the Stroop color reading test is the prefrontal cortex [27]. From our results, we can infer that baseline olfactory dysfunction is closely linked to the early presence of Lewy bodies in the frontotemporal regions, which leads to dysfunction in naming and frontal execution in PD. Pathological studies are necessary to investigate the timing and preferential site for cortical involvement of Lewy bodies according to olfactory dysfunction.
We found that PD patients with severe olfactory dysfunction at the time of PD diagnosis had a higher rate of conversion to dementia than other PD patients, independent of age, sex, and baseline motor and cognitive functions. Consistent with our study, previous studies have reported that baseline hyposmia in PD patients is an independent risk factor for the subsequent development of MCI [6] and dementia [3]. Additionally, PD patients with baseline hyposmia had a characteristic cerebral metabolic decline that was identical to that in PD dementia [8]. Olfactory dysfunction was also closely associated with cognitive decline, even in dementia-free subjects [28] and in patients with amnestic MCI and Alzheimer’s disease (AD) [29]. Worse olfaction may reflect early and severe extranigral Lewy body pathology in the cortical areas, which could accelerate the progression of cognitive decline and conversion to dementia [6]. Early damage to the forebrain cholinergic system could be another explanation for the association between olfactory dysfunction and cognitive decline. Patients with PD show substantial neuronal loss in the nucleus basalis of Meynert [30]. Odor identification test scores have been shown to be positively correlated with acetylcholinesterase activity in the limbic area [23]. PET studies have revealed widespread and profound cholinergic dysfunction in PD dementia [31]. If we can identify a temporal relationship between olfactory impairment and cholinergic dysfunction, it will enable us to elucidate the role of olfactory dysfunction in cognitive decline. Last, co-accumulation of AD neuropathologies such as amyloid plaque and neurofibrillary tangles can link olfactory dysfunction and cognitive decline. Tau-related pathology has been found in the anterior olfactory nucleus in PD [32]. Olfactory impairment has been shown to be associated with atrophy in AD signature areas in dementia-free older adults [33]. Concomitant AD neuropathologies have been found to be associated with rapid cognitive decline in Lewy body disorders [34]. Since patients in the anosmic PD group in this study had a higher age of onset and proportion of MCI than the other groups, AD co-pathology may have accelerated cognitive decline and dementia conversion in the anosmic PD group. It is necessary to investigate the burden of amyloid-β burden according to the baseline olfactory function in PD.
Olfactory dysfunction was not associated with baseline parkinsonian motor severity, putaminal DAT activity, the longitudinal LED increment, or long-term motor complications. A previous study reported that PD patients stratified by olfactory tertile had similar baseline UPDRS motor scores and changes in UPDRS scores [6]. The absence of an effect of levodopa use on olfactory performance is additional indirect evidence of no relationship between dopamine and olfaction [35]. Studies using DAT imaging showed no association between putaminal DAT activity and olfactory function [11]. Two studies have reported contradictory results, suggesting that olfactory dysfunction is correlated with higher motor severity and LED, freezing of gait, and putaminal DAT binding [7,36]. However, these studies enrolled PD patients with a wide range of disease durations and did not adjust for the disease duration, which was closely related to motor severity. It would be difficult to investigate the independent relationship between olfactory function and motor function without controlling for this factor. These results suggest that PD patients have similar nigral pathologic burdens regardless of olfactory dysfunction. In terms of motor complications such as WO and LID, low putaminal DAT activity [37], disease severity [38], and a high dose of levodopa39 are risk factors for WO and LID, none of which were associated with baseline olfactory dysfunction in this study. Fullard et al. [6] also found that olfactory dysfunction was not associated with putaminal DAT activity, UPDRS motor score, or disease progression. Therefore, we can infer that early and severe olfactory dysfunction and possible extranigral pathology are unlikely to be associated with presynaptic nigrostriatal degeneration and long-term synaptic plasticity [40].
This study has several limitations. First, we evaluated olfactory function only based on the aspect of identification, which is thought to require cognitive memory processing [41], even though olfaction can be assessed using various methods, including odor discrimination and detection threshold tasks. Second, we did not monitor UPDRS motor scores regularly, and we did not perform regular neuropsychological tests. Instead, we calculated the LED increment, which has not been validated as a marker reflective of motor progression and monitored the occurrence of motor and cognitive complications. Investigating the association between olfactory dysfunction and the progression of motor and cognitive function is important for the elucidation of the role of olfactory impairment and the pathological spread of nigral and extranigral pathologies in PD. Third, we did not confirm the associations between the nigral and extranigral burden of PD pathology and olfactory dysfunction. Although we used cortical Lewy body involvement to propose a relationship between olfactory and cognitive dysfunction, other possible mechanisms, such as cholinergic dysfunction or concomitant Alzheimer’s disease pathology, could also be associated. Future studies are necessary to investigate these factors to identify the role of olfaction in cognitive decline in PD. Last, this is a retrospective cohort study, which has some disadvantages; the lack of uniformity in baseline evaluations and differential losses to follow-up in some patients may have introduced bias, and the collection of data at different intervals prevented us from identifying an exposed cohort and comparison group appropriately. A prospective study is necessary to clearly identify whether olfactory deficits are an independent risk factor for cognitive complications. Despite these limitations, in this study, we used a large sample of PD patients and investigated motor and cognitive dysfunction in the aspects of baseline function, long-term outcomes, and DAT activity in the same cohort. This resulted in reliable and generalizable results with statistical consistency.
We demonstrated that baseline olfactory dysfunction was not associated with motor deficits and complications, but it was associated with cognitive dysfunction and dementia conversion. This suggests that early and severe olfactory impairment is likely to be associated with early cortical involvement, probably in the frontotemporal region, and the rapid spreading of Lewy body pathology. Olfactory dysfunction can be a useful biomarker when assessing patients with PD who are at risk of early development of cognitive dysfunction and dementia.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.14802/jmd.19082.
Supplementary Table 1
Correlation between olfactory function (CCSIT score) and neuropsychological performance
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.
Ethical Standard
All procedures involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards.
Author Contributions
Conceptualization: Han Soo Yoo and Phil Hyu Lee. Data curation: Han Soo Yoo and Phil Hyu Lee. Formal analysis: Han Soo Yoo and Seok Jong Chung. Funding acquisition: Phil Hyu Lee. Investigation: Han Soo Yoo, Seok Jong Chung, and Phil Hyu Lee. Methodology: Han Soo Yoo, Seok Jong Chung, and Byoung Seok Ye. Project administration: Young H. Sohn and Phil Hyu Lee. Supervision: Byoung Seok Ye, Young H. Sohn, and Phil Hyu Lee. Visualization: Han Soo Yoo and Seok Jong Chung. Writing—original draft: Han Soo Yoo, Seok Jong Chung, and Phil Hyu Lee. Writing—review & editing: all authors.
Acknowledgements
The authors are grateful to Jungsu S. Oh and Jae Seung Kim (Department of Nuclear Medicine, Asan Medical Center, University of Ulsan College of Medicine) for the quantitative analyses of the 18F-FP-CIT PET images.
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (grant number: NRF-2016R1A2A2A05920131).