Articles
- Page Path
- HOME > J Mov Disord > Volume 13(3); 2020 > Article
-
Original Article
Therapeutic Effect of Levodopa/Carbidopa/Entacapone on Sleep Disturbance in Patients with Parkinson’s Disease -
Kye Won Park1
 , Sungyang Jo1
, Sungyang Jo1 , Seung Hyun Lee1
, Seung Hyun Lee1 , Yun Su Hwang1
, Yun Su Hwang1 , Dagyo Lee1
, Dagyo Lee1 , Ho-Sung Ryu2
, Ho-Sung Ryu2 , Sun Ju Chung1
, Sun Ju Chung1
-
Journal of Movement Disorders 2020;13(3):205-212.
DOI: https://doi.org/10.14802/jmd.20055
Published online: September 9, 2020
1Department of Neurology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
2Department of Neurology, Kyungpook National University Hospital, Daegu, Korea
- Corresponding author: Sun Ju Chung, MD, PhD Department of Neurology, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul, Korea / Tel: +82-2- 3010-3440 / Fax: +82-2-474-4691 / E-mail: sjchung@amc.seoul.kr
Copyright © 2020 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objective
- To investigate the efficacy of levodopa/carbidopa/entacapone (LCE) at bedtime for treating sleep disturbance in patients with Parkinson’s disease (PD) with motor fluctuations.
-
Methods
- Participants included 128 PD patients with motor fluctuations. All patients were assessed for motor, nonmotor, and sleep-specific symptoms using the United Parkinson’s Disease Rating Scale (UPDRS), the Korean version of the Nonmotor Symptom Scale, the Parkinson’s Disease Sleep Scale (PDSS), the Epworth Sleepiness Scale, and the Rapid Eye Movement Sleep Behavior Disorder Screening Questionnaire (RBDSQ). We compared the baseline characteristics of patients with sleep disturbance (PDSS score < 120) and those without sleep disturbance (PDSS score ≥ 120). Thirty-nine patients with sleep disturbance who agreed to take LCE at bedtime completed 3-month follow-ups. We analyzed changes in the scores of motor, nonmotor, and sleep symptom scales over the 3 months.
-
Results
- PD patients with sleep disturbance were at more advanced disease stages and had more severe motor, nonmotor, and sleep symptoms than those without sleep disturbance. Patients who took LCE at night showed improvements in motor (UPDRS part III, p = 0.007) and sleep symptoms (total PDSS, p < 0.001). Sleep features that benefitted from LCE included not only nocturnal motor components but also insomnia (PDSS items 2 and 3, p = 0.005 and p < 0.001) and rapid eye movement behavior disorder (PDSS item 6, p = 0.002; and RBDSQ, p < 0.001).
-
Conclusion
- The use of LCE at bedtime may be a useful treatment for sleep disturbance in advanced PD patients with motor fluctuations.
- Patients
- PD patients were diagnosed based on the United Kingdom Parkinson’s Disease Society Brain Bank diagnostic criteria and were recruited from March 2016 to February 2018. The inclusion criteria were as follows: 1) age between 20–79 years old, 2) Hoehn and Yahr (H&Y) stage 1–4, 3) presence of wearing-off phenomenon, 4) absence of significant cognitive impairment as determined by a Montreal Cognitive Assessment (MoCA) score ≥ 15 [7], 5) absence of significant depression as determined by a Geriatric Depression Scale (GDS) score ≤ 24 [8], and 6) consent to participate in the study. Patients with atypical parkinsonism, including multiple system atrophy, corticobasal syndrome, and progressive supranuclear palsy, were excluded from the study, as were patients who previously experienced side effects due to LCE. This study was approved by the local Institutional Review Board (IRB) (IRB number: 2015-0241). Written informed consent was obtained from all participants.
- Clinical assessment
- All patients were assessed using the United Parkinson’s Disease Rating Scale (UPDRS), H&Y stage, the Schwab and England Activities of Daily Living (SE-ADL) scale, the Parkinson’s Disease Sleep Scale (PDSS) [9], the Epworth Sleepiness Scale (ESS), the Korean versions of RBD screening questionnaire (RBDSQ), MoCA, the Parkinson’s Disease Quality of Life Questionaire-39 (PDQ-39), the Nonmotor Symptom Scale for Parkinson’s Disease (NMSS) [10], and the GDS at baseline. All scales were rated during the ‘on’ state, i.e., at a time when the patient reported optimal or partial relief of parkinsonian symptoms due to dopaminergic medications. The total PDSS score was used to evaluate the overall quality of sleep, and a total PDSS score below 120 indicated sleep disturbance [9]. The 15 items of the PDSS that cover the entire spectrum of sleep disturbance in PD are as follows: overall quality of night sleep (item 1), insomnia sleep onset and maintenance insomnia (items 2 and 3), nocturnal restlessness (items 4 and 5), nocturnal psychosis (items 6 and 7), nocturia (items 8 and 9), nocturnal motor symptoms (items 10–13), sleep refreshment (item 14), and daytime dozing (item 15).
- Among patients experiencing sleep disturbance (PDSS score < 120), those who agreed to take additional levodopa were started on LCE (Stalevo®, Novartis Pharmaceuticals Corp., East Hanover, NJ, USA) at bedtime for 3 months, with the dosage adjusted based on each patient’s previous levodopa-equivalent daily dose (LEDD). The dose of anti-Parkinson drugs and (if applicable) the doses of hypnotics, anxiolytics, antipsychotics, and antidepressants were fixed until the end of the 3-month follow-up. None of the patients were taking nighttime levodopa prior to this trial. Each patient underwent a follow-up assessment using the scales described above, including the individual items of the PDSS, at 1 and 3 months to assess the interval change in the scale scores.
- Statistical analysis
- We compared the clinical features of PD patients experiencing sleep disturbance (PDSS score < 120) to those of patients who were not experiencing sleep disturbance (PDSS score ≥ 120) at baseline according to various motor, nonmotor, and sleep features of PD. Second, we analyzed changes in sleep and other motor and nonmotor PD features caused by the use of LCE at bedtime during the 3-month follow-up. Last, we compared baseline features between PD patients who experienced overall sleep improvement (PDSS improvement > 15%) due to the use of LCE at bedtime (i.e., responders) and those who did not experienced overall sleep improvement (i.e., nonresponders). Continuous variables were compared using Student’s t-tests, and categorical variables were compared using chi-square tests. A repeated measures analysis of variance was used to compare changes in scores on each scale at baseline, 1 month, and 3 months. A p value < 0.05 was considered statistically significant. For the analysis of individual PDSS items, a false discovery rate (FDR) was applied to correct for multiple comparisons.
MATERIALS & METHODS
- Clinical characteristics of PD patients who do and do not experience sleep disturbance
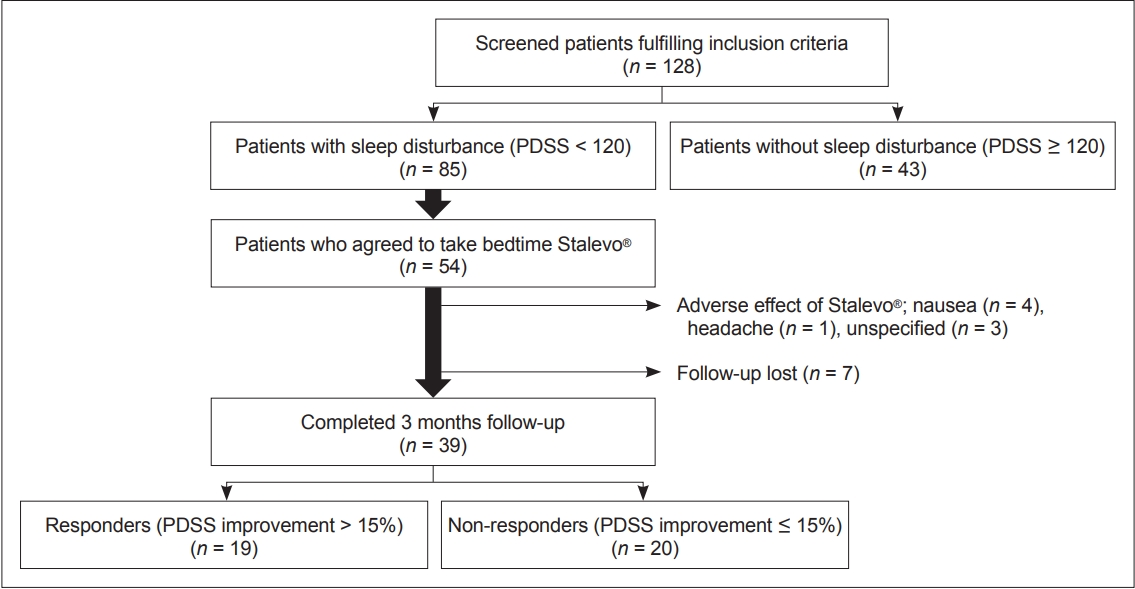
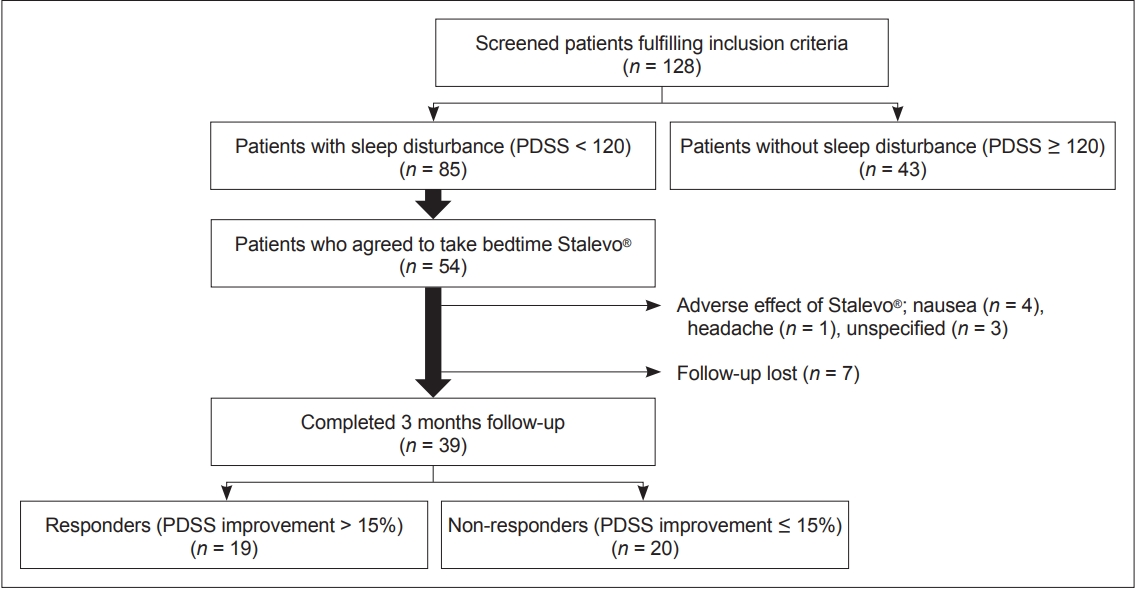
- A total of 128 PD patients participated in the study. Among them, 85 (66%) patients experienced sleep disturbance (PDSS score < 120), and 43 (34%) did not experience sleep disturbance (PDSS score ≥ 120) (Figure 1, Table 1). Compared to PD patients who did not experience sleep disturbance, those who did had longer mean disease durations (p = 0.015), were at more advanced H&Y stages (p = 0.045), and had higher LEDD (p = 0.031) at baseline. They also had worse relative baseline scores on the full UPDRS (p = 0.005), UPDRS part IV (p < 0.001), PDQ-39 (p < 0.001), NMSS (p < 0.001), and GDS (p < 0.001). Overall, these results are indicative of worse motor and nonmotor symptoms in patients experiencing sleep disturbance. In terms of sleep scales, scores on all 15 PDSS items (data not shown) as well as the ESS (p < 0.001) and the RBDSQ (p = 0.005) were lower in patients experiencing sleep disturbance. This indicates that all components of sleep are affected in PD patients experiencing sleep disturbance.
- Changes in motor, nonmotor, and sleep features of PD due to the use of LCE at bedtime
- Among the 85 patients with sleep disturbance, 54 patients agreed to take LCE at bedtime in addition to their regular daytime medication (Figure 1). Thirty-nine patients (72%) completed the 3-month follow-up. Eight patients withdrew due to various side effects from LCE, and seven were lost to follow-up for unspecified reasons.
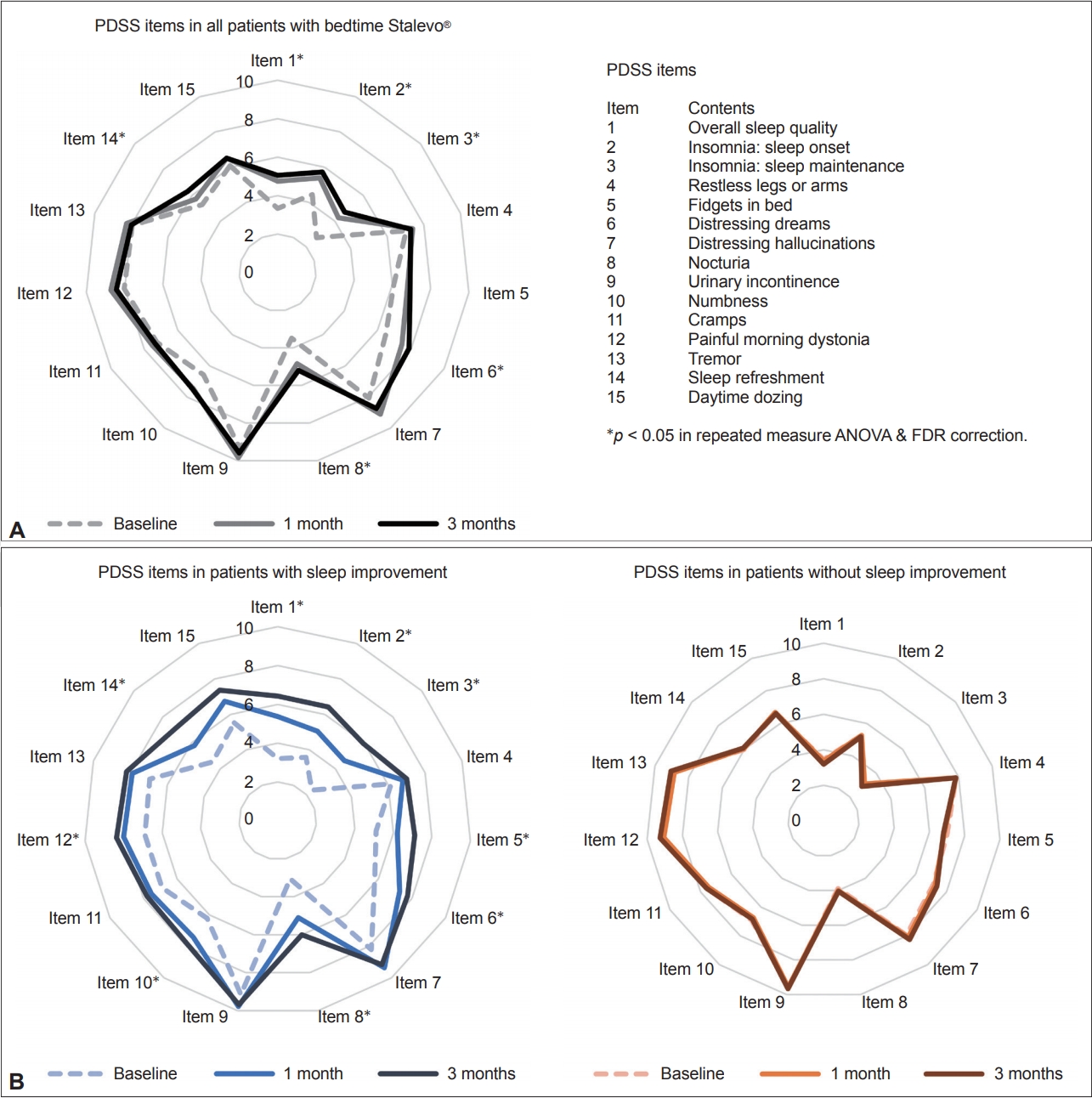
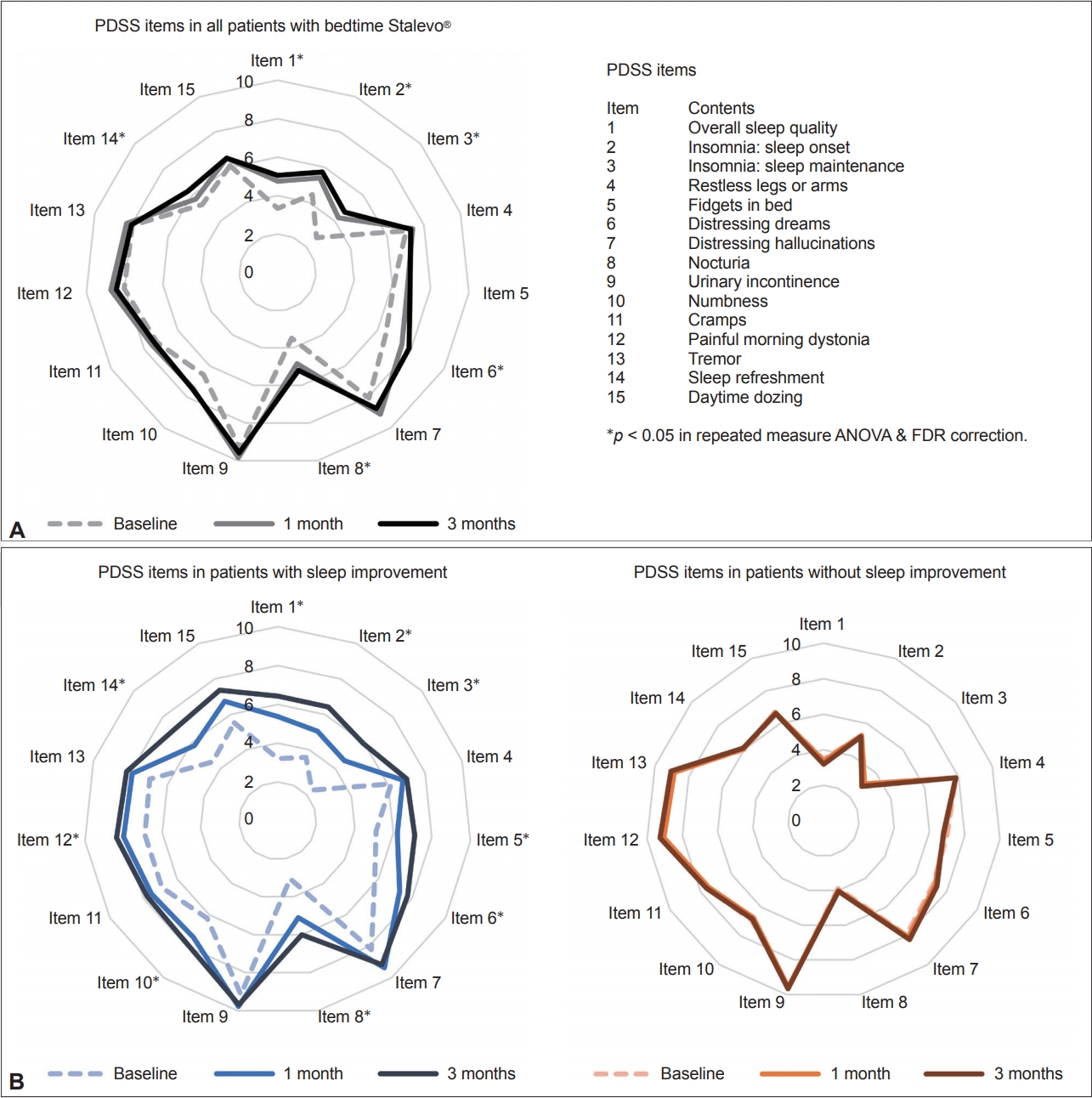
- As expected, patients who took an additional dose of LCE showed statistically significant improvements in motor features, reflected by the improvement of total UPDRS (p = 0.017), UPDRS part III (p = 0.007), and UPDRS part IV (p = 0.006) scores (Table 2). There was no overall improvement in nonmotor symptom severity, as shown by NMSS scores (p = 0.059) (Table 2). In terms of sleep, patients showed an overall improvement in sleep quality, as shown by an improvement in the total PDSS score (p < 0.001), scores for PDSS item 1 (overall sleep quality, p < 0.001), and scores for PDSS item 14 (sleep refreshment, p = 0.018) (Table 2, Figure 2A). Patients also showed improvements in sleep onset (PDSS item 2, p = 0.005), sleep maintenance (PDSS items 3, p < 0.001), distressing dreams (PDSS item 6, p = 0.002), nocturia (PDSS item 8, p < 0.001), and RBD (RBDSQ, p < 0.001) (Table 2, Figure 2A).
- Clinical comparison of PD patients who did and did not experience improved sleep due to the use of LCE at bedtime
- Although patients who used LCE at bedtime showed an overall improvement in sleep, the degree of improvement was highly heterogeneous (the change in PDSS scores ranged from -46% to 84%). For this reason, we divided the patients into two groups according to their degree of improvement at 3 months: patients who had PDSS improvements of over 15% (i.e., responders, n = 19, average PDSS increment 34%) and patients who had improvements of less than 15% (i.e., nonresponders, n = 20, average PDSS increment -1.7%) (Figure 1). At baseline, responders had worse PDSS scores than nonresponders (p = 0.003), but there was no difference in other motor, nonmotor, or sleep features of PD except for slightly lower ADL scores in responders (Table 3). For individual PDSS items, responders had worse scores only on PDSS item 12 (morning off dystonia, p = 0.002) after FDR correction.
- We also assessed changes in motor, nonmotor, and sleep features in PD for each group during the 3-month follow-up (Table 3, Figure 2B). Responders showed improvement in most aspects of sleep, including total PDSS scores, scores on 9 of the 15 PDSS items (after FDR correction), and RBDSQ scores. Notably, nonresponders showed significant improvements in RBDSQ scores at the 3-month follow-up (p = 0.007), although no other features showed improvement over this period (Table 3).
RESULTS
- The present study investigated the characteristics of PD patients who have sleep disturbance, identified clinical features that could be improved with the use of LCE at bedtime, and characterized the patients whose sleep would benefit from the use of LCE at bedtime. First, it was found that PD patients with poorer sleep quality had more advanced disease stages and more severe symptoms, as measured by motor, nonmotor, and sleep scales, than those with better sleep quality. Second, LCE improved sleep by improving nonmotor as well as motor symptoms. Specifically, sleep onset, sleep maintenance, and RBD were improved by the use of LCE. We also found that those with lower PDSS scores at baseline responded more favorably to LCE in terms of sleep improvements.
- The disruption of sleep and circadian rhythms is highly prevalent in patients with PD [4,11]. Interestingly, impaired sleep was one of the first recognized symptoms of PD, as stated in the earliest description of the disease by James Parkinson in his 1817 essay “An Essay on the Shaking Palsy.” [11] Although there have been tremendous advancements in treating the motor symptoms of PD over the last two centuries, little advancement has been made in regards to treating sleep disturbance in patients with PD. Nevertheless, sleep disturbance is one of the most troublesome and irritating symptoms for patients with PD. It has been shown that poor sleep quality in patients with PD is associated with diminished quality of life, depression, increased burden for caregivers, and more advanced motor symptoms [12-14]. Patients from our study population who had poor sleep quality had worse motor, nonmotor, and sleep symptoms (Table 1). Notably, the GDS score was significantly higher in patients with lower PDSS scores even though we excluded patients with overt depression (GDS > 24) in the screening, thus reflecting the strong association between depression and sleep disturbance in PD. Thus, the treatment of patients with PD should take sleep symptoms into account to optimally improve quality of life.
- We found that the use of levodopa at bedtime may improve sleep quality in patients with advanced PD. Dopamine, the key neurotransmitter that is deficient in the PD brain, is an emerging key factor for circadian regulation [6,11,15]. Studies have shown the existence of dopamine-mediated circadian-like activity in major areas of the central nervous system, including the striatum, olfactory bulb, midbrain, hypothalamus, and retina [1,6]. The factors for sleep disturbances in PD can be categorized into three groups: 1) primary sleep disorders in PD due to altered circadian regulation, including insomnia, RBD, and EDS; 2) secondary sleep disorders in PD due to nocturnal motor symptoms, including nocturnal akinesia and morning dystonia; and 3) other miscellaneous causes, including nocturnal psychiatric symptoms and treatment-related nocturnal disturbances [4,16,17]. Traditionally, levodopa has been recommended for secondary sleep disorders due to motor symptoms worsening at night [4]. Given the recent finding that dopamine is a key neurotransmitter for circadian regulation, its utility for treating primary sleep disorders in PD should be reconsidered. We recruited advanced PD patients with motor fluctuations to encompass both the primary and secondary sleep disorders of PD. The triple combination drug Stalevo® (levodopa/carbidopa/entacapone) was chosen because the agent is indicated for advanced PD patients with motor fluctuations. In addition, few trials have been conducted to examine the efficacy of such triple combination regimens for sleep disturbance in patients with PD. Our results showed a fair amount of improvement not only in nocturnal motor symptoms but also in RBD scores, even in the subgroup that did not display a significant improvement in total PDSS scores. There are several other reports showing improvements in RBD due to levodopa therapy [18-20]. These findings reflect the possible role of levodopa as a treatment option for all types of sleep disturbance in PD.
- Nevertheless, for several reasons, we suggest that more well-designed trials are warranted to examine the efficacy of levodopa, to determine the optimal regimen of levodopa and to identify potential better responders of levodopa for the treatment of sleep disturbance in PD. First, the dropout rate of our study was relatively high (15 out of 54, 28%). This could have biased the results due to the exclusion of poor responders. Second, contrary to our study, some previous trials that studied the efficacy of using levodopa at night did not identify objective improvements in the sleep parameters of PD patients [21,22]. Unlike our study, these trials used controlled-releasing levodopa formula. The difference in the peak dose effect might have affected the controversial clinical results. The efficacy of different formulas or regimens of levodopa for sleep disturbance in PD has yet to be elucidated. Third, the patients’ responses to levodopa were highly heterogeneous in our study population. We tried to determine the clinical factors that affect responses to levodopa by comparing the baseline characteristics of responders and nonresponders. However, we only found that those who showed improvement in sleep quality as a result of LCE had relatively worse PDSS scores at baseline. Additional studies with more objective parameters (e.g., polysomnographic parameters, genetic variants of circadian and clock genes) may provide a better understanding of who would show improvements in sleep symptoms due to the use of levodopa at bedtime.
- There are several limitations to this study. Most importantly, the study was an open-label study without a placebo group. The size of the placebo effect on the overall improvement resulting from LCE is hard to quantify. Furthermore, our study was based on clinical scales that were subjectively completed by clinicians and patients. Although these scales are well validated and reliable, our study lacks objective polysomnographic parameters to evaluate changes in sleep.
- In conclusion, we investigated the characteristics of PD patients with sleep disturbance and their clinical response to the use of LCE at bedtime. Sleep disturbance is associated with more severe motor and nonmotor symptoms, and the use of LCE at bedtime is a possible treatment option for patients with PD who are experiencing sleep disturbance. Further clinical trials with objective sleep parameters and various regimens of levodopa to treat sleep disturbance of PD should be conducted.
DISCUSSION
-
Conflicts of Interest
The authors have no financial conflicts of interest.
-
Ethical Standard
All procedures performed in studies involving human participants were conducted in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all the patients included in the study
-
Author Contributions
Conceptualization: Kye Won Park, Ho-Sung Ryu, Sun Ju Chung. Data curation: Kye Won Park, Dagyo Lee. Formal analysis: Kye Won Park. Funding acquisition: Sun Ju Chung. Investigation: Kye Won Park, Dagyo Lee, HoSung Ryu. Methodology: Kye Won Park, Ho-Sung Ryu, Sun Ju Chung. Project administration: Sun Ju Chung. Resources: Kye Won Park, Dagyo Lee, HoSung Ryu, Sun Ju Chung. Software: Kye Won Park. Supervision: Sun Ju Chung. Validation: Sungyang Jo, Seung Hyun Lee, Yun Su Hwang. Visualization: Kye Won Park. Writing—original draft: Kye Won Park. Writing—review & editing: Sungyang Jo, Seung Hyun Lee, Yun Su Hwang, Dagyo Lee, Sun Ju Chung.
Notes
- This study was supported by a grant from Novartis Korea Ltd. (CELC200DKR04T).
Acknowledgments
| PD with sleep disturbance (PDSS < 120) (n = 85) | PD without sleep disturbance (PDSS ≥ 120) (n = 43) | p value | |
|---|---|---|---|
| Age (years) | 65.9 ± 7.9 | 65.4 ± 7.9 | 0.700 |
| Age at onset (years) | 56.6 ± 8.4 | 58.3 ± 8.5 | 0.297 |
| Disease duration (years) | 9.3 ± 5.0 | 7.1 ± 4.5 | 0.015* |
| Female | 50 (59) | 19 (44) | 0.135 |
| Patients on hypnotics/anxiolytics | 14 (16) | 9 (21) | 0.535 |
| Patients on antipsychotics | 0 (0) | 2 (5) | 0.111 |
| Patients on antidepressants | 4 (5) | 0 (9) | 0.300 |
| H&Y stage | 2.6 ± 0.6 | 2.3 ± 0.5 | 0.045* |
| LEDD (mg) | 903 ± 321 | 770 ± 335 | 0.031* |
| MoCA | 24.7 ± 3.9 | 24.7 ± 3.1 | 0.863 |
| UPDRS, total | 45.1 ± 18.8 | 34.0 ± 13.9 | 0.005* |
| UPDRS, part I | 5.5 ± 4.9 | 4.6 ± 4.1 | 0.319 |
| UPDRS, part II | 16.0 ± 11.6 | 19.4 ± 11.4 | 0.119 |
| UPDRS, part III | 26.9 ± 12.2 | 25.5 ± 10.9 | 0.525 |
| UPDRS, part IV | 4.7 ± 2.8 | 1.8 ± 2.1 | < 0.001* |
| SE-ADL | 82.3 ± 10.7 | 85.8 ± 10.1 | 0.090 |
| PDQ-39 | 41.8 ± 22.2 | 22.4 ± 20.9 | < 0.001* |
| PDSS | 95.8 ± 18.8 | 134.5 ± 7.6 | < 0.001* |
| ESS | 8.1 ± 4.2 | 5.2 ± 2.4 | < 0.001* |
| RBDSQ | 5.9 ± 2.8 | 4.4 ± 3.0 | 0.005* |
| NMSS | 57.6 ± 31.8 | 30.0 ± 23.1 | < 0.001* |
| GDS | 13.7 ± 6.4 | 7.8 ± 6.9 | < 0.001* |
Data are shown as the mean ± standard deviation or n (%).
* statistically significant.
PD: Parkinson’s disease, H&Y: Hoehn and Yahr, LEDD: levodopa-equivalent daily dose, MoCA: Montreal Cognitive Assessment, UPDRS: Unified Parkinson’s Disease Rating Scale, SE-ADL: Schwab and England Activities of Daily Living, PDQ-39: Parkinson’s Disease Quality of Life Questionaire-39, PDSS: Parkinson’s Disease Sleep Scale, ESS: Epworth Sleepiness Scale, RBDSQ: Rapid Eye Movement Behavior Disorder Screening Questionnaire, NMSS: the Nonmotor Symptom Scale, GDS: Geriatric Depression Scale.
| Baseline | 1 month | 3 months | p value | |
|---|---|---|---|---|
| H&Y stage | 2.6 ± 0.5 | 2.6 ± 0.5 | 2.6 ± 0.5 | 0.767 |
| UPDRS, total | 49.3 ± 18.8 | 45.7 ± 17.2 | 44.1 ± 19.7 | 0.017* |
| UPDRS, part I | 3.1 ± 2.0 | 2.9 ± 1.7 | 3.4 ± 2.0 | 0.166 |
| UPDRS, part II | 11.2 ± 6.4 | 10.6 ± 6.0 | 11.6 ± 6.9 | 0.556 |
| UPDRS, part III | 29.3 ± 11.4 | 27.0 ± 10.5 | 25.9 ± 10.3 | 0.007* |
| UPDRS, part IV | 5.7 ± 2.9 | 5.1 ± 2.9 | 5.0 ± 3.0 | 0.006* |
| SE-ADL | 80.0 ± 11.2 | 79.6 ± 11.0 | 79.4 ± 11.4 | 0.442 |
| PDQ-39 | 44.5 ± 23.5 | 44.0 ± 24.5 | 47.1 ± 27.5 | 0.348 |
| PDSS | 92.2 ± 19.6 | 102.5 ± 21.3 | 105.2 ± 20.9 | < 0.001* |
| ESS | 3.3 ± 4.4 | 7.5 ± 4.3 | 7.4 ± 4.5 | 0.260 |
| RBDSQ | 6.4 ± 2.8 | 4.7 ± 3.0 | 4.3 ± 2.5 | < 0.001* |
| NMSS | 69.5 ± 33.7 | 63.1 ± 31.4 | 63.6 ± 34.6 | 0.059 |
Data are shown as the mean ± standard deviation.
* statistically significant by repeated measure ANOVA.
LCE: levodopa/carbidopa/entacapone, H&Y: Hoehn and Yahr, UPDRS: Unified Parkinson’s Disease Rating Scale, SE-ADL: Schwab and England Activities of Daily Living, PDQ-39: Parkinson’s Disease Quality of Life Questionaire-39, PDSS: Parkinson’s Disease Sleep Scale, ESS: Epworth Sleepiness Scale, RBDSQ: Rapid Eye Movement Behavior Disorder Screening Questionnaire, NMSS: the Nonmotor Symptom Scale.
| Responders (n = 19) (PDSS improvement > 15%) | Nonresponders (n = 20) (PDSS improvement ≤ 15%) | p value | |||||
|---|---|---|---|---|---|---|---|
| Levodopa dose of LCE (mg) | 150 [100–200] | 150 [100–200] | 0.667 | ||||
| Age (years) | 66.0 ± 6.7 | 64.5 ± 9.4 | 0.477 | ||||
| Age at onset (years) | 57.3 ± 8.6 | 55.6 ± 8.9 | 0.183 | ||||
| Disease duration (years) | 8.7 ± 5.9 | 8.9 ± 3.7 | 0.294 | ||||
| Female | 13 (68) | 12 (60) | 0.741 | ||||
| Patients on hypnotics/anxiolytics | 4 (21) | 5 (25) | 1.000 | ||||
| Patients on antipsychotics | 0 (0) | 0 (0) | 1.000 | ||||
| Patients on antidepressants | 1 (5) | 1 (5) | 1.000 | ||||
| H&Y stage | 2.6 ± 0.2 | 2.6 ± 0.3 | 0.587 | ||||
| LEDD (mg) | 875 ± 347 | 951 ± 297 | 0.467 | ||||
| MoCA | 24.6 ± 4.1 | 25.2 ± 3.2 | 0.512 | ||||
| Baseline | 3 months | p value† | Baseline | 3 months | p value† | p value‡ | |
| UPDRS, total | 53.1 ± 20.0 | 44.3 ± 21.8 | 0.002* | 45.6 ± 17.2 | 43.9 ± 18.0 | 0.589 | 0.126 |
| UPDRS, part I | 3.7 ± 2.2 | 3.5 ± 1.9 | 0.170 | 2.5 ± 1.7 | 3.2 ± 1.9 | 0.021 | 0.058 |
| UPDRS, part II | 12.7 ± 6.7 | 12.0 ± 7.0 | 0.192 | 9.8 ± 6.0 | 11.2 ± 6.8 | 0.191 | 0.158 |
| UPDRS, part III | 30.2 ± 11.9 | 26.3 ± 10.3 | 0.001* | 28.4 ± 11.0 | 25.6 ± 10.6 | 0.127 | 0.443 |
| UPDRS, part IV | 5.9 ± 3.3 | 5.0 ± 3.3 | 0.007* | 5.4 ± 2.5 | 4.9 ± 2.6 | 0.225 | 0.572 |
| SE-ADL | 76.8 ± 11.5 | 77.8 ± 11.3 | 0.270 | 83.0 ± 10.3 | 80.7 ± 11.5 | 0.119 | 0.049* |
| PDQ-39 | 46.5 ± 27.5 | 45.8 ± 30.0 | 0.783 | 42.5 ± 19.5 | 48.2 ± 25.5 | 0.219 | 0.494 |
| PDSS | 83.6 ± 19.6 | 112.2 ± 23.1 | < 0.001* | 100.3 ± 16.5 | 98.6 ± 16.5 | 0.576 | 0.003* |
| ESS | 8.0 ± 3.8 | 6.1 ± 3.4 | 0.362 | 8.1 ± 4.8 | 8.6 ± 5.1 | 0.545 | 0.728 |
| RBDSQ | 6.0 ± 2.4 | 3.7 ± 2.1 | 0.001* | 6.7 ± 3.2 | 4.8 ± 2.6 | 0.007* | 0.686 |
| NMSS | 74.3 ± 35.6 | 59.0 ± 32.8 | 0.004* | 64.9 ± 31.9 | 67.9 ± 36.5 | 0.378 | 0.321 |
Data are shown as the median [range], mean ± standard deviation, or n (%).
* statistically significant,
† comparison between baseline, 1 month, and 3 months follow-up in each group by repeated measure analysis of variance,
‡ comparison between responders and nonresponders at baseline.
LCE: levodopa/carbidopa/entacapone, H&Y: Hoehn and Yahr, LEDD: levodopa-equivalent daily dose, MoCA: Montreal Cognitive Assessment, UPDRS: Unified Parkinson’s Disease Rating Scale, SE-ADL: Schwab and England Activities of Daily Living, PDQ-39: Parkinson’s Disease Quality of Life Questionaire-39, PDSS: Parkinson’s Disease Sleep Scale, ESS: Epworth Sleepiness Scale, RBDSQ: Rapid Eye Movement Behavior Disorder Screening Questionnaire, NMSS: the Nonmotor Symptom Scale.
- 1. Verbaan D, van Rooden SM, Visser M, Marinus J, van Hilten JJ. Nighttime sleep problems and daytime sleepiness in Parkinson’s disease. Mov Disord 2008;23:35–41.ArticlePubMed
- 2. Comella CL. Sleep disorders in Parkinson’s disease: an overview. Mov Disord 2007;22(Suppl 17):S367–S373.ArticlePubMed
- 3. De Pablo-Fernández E, Courtney R, Warner TT, Holton JL. A histologic study of the circadian system in Parkinson disease, multiple system atrophy, and progressive supranuclear palsy. JAMA Neurol 2018;75:1008–1012.ArticlePubMedPMC
- 4. Barone P, Amboni M, Vitale C, Bonavita V. Treatment of nocturnal disturbances and excessive daytime sleepiness in Parkinson’s disease. Neurology 2004;63(8 Suppl 3):S35–S38.Article
- 5. Loddo G, Calandra-Buonaura G, Sambati L, Giannini G, Cecere A, Cortelli P, et al. The treatment of sleep disorders in Parkinson’s disease: from research to clinical practice. Front Neurol 2017;8:42.ArticlePubMedPMC
- 6. Korshunov KS, Blakemore LJ, Trombley PQ. Dopamine: a modulator of circadian rhythms in the central nervous system. Front Cell Neurosci 2017;11:91.ArticlePubMedPMC
- 7. Kim JI, Sunwoo MK, Sohn YH, Lee PH, Hong JY. The MMSE and MoCA for screening cognitive impairment in less educated patients with Parkinson’s disease. J Mov Disord 2016;9:152–159.ArticlePubMedPMCPDF
- 8. Jung IK, Kwak DI, Joe SH, Lee HS. A preliminary study on standardization of Korean Form of Geriatric Depression Scale (KGDS). J Korean Neuropsychiatr Assoc 1998;37:340–351.
- 9. Chaudhuri KR, Pal S, DiMarco A, Whately-Smith C, Bridgman K, Mathew R, et al. The Parkinson’s disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2002;73:629–635.ArticlePubMedPMC
- 10. Koh SB, Kim JW, Ma HI, Ahn TB, Cho JW, Lee PH, et al. Validation of the Korean-version of the nonmotor symptoms scale for Parkinson’s disease. J Clin Neurol 2012;8:276–283.ArticlePubMedPMC
- 11. Videnovic A, Golombek D. Circadian and sleep disorders in Parkinson’s disease. Exp Neurol 2013;243:45–56.ArticlePubMed
- 12. Kay DB, Tanner JJ, Bowers D. Sleep disturbances and depression severity in patients with Parkinson’s disease. Brain Behav 2018;8:e00967.ArticlePubMedPMC
- 13. Smith MC, Ellgring H, Oertel WH. Sleep disturbances in Parkinson’s disease patients and spouses. J Am Geriatr Soc 1997;45:194–199.ArticlePubMed
- 14. Rodrigues TM, Castro Caldas A, Ferreira JJ. Pharmacological interventions for daytime sleepiness and sleep disorders in Parkinson’s disease: systematic review and meta-analysis. Parkinsonism Relat Disord 2016;27:25–34.ArticlePubMed
- 15. Videnovic A, Willis GL. Circadian system - a novel diagnostic and therapeutic target in Parkinson’s disease? Mov Disord 2016;31:260–269.ArticlePubMedPMC
- 16. Patke A, Young MW, Axelrod S. Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol 2020;21:67–84.ArticlePubMed
- 17. Xue F, Wang FY, Mao CJ, Guo SP, Chen J, Li J, et al. Analysis of nocturnal hypokinesia and sleep quality in Parkinson’s disease. J Clin Neurosci 2018;54:96–101.ArticlePubMed
- 18. Yamauchi K, Takehisa M, Tsuno M, Kaneda Y, Taniguchi T, Ohno H, et al. Levodopa improved rapid eye movement sleep behavior disorder with diffuse Lewy body disease. Gen Hosp Psychiatry 2003;25:140–142.ArticlePubMed
- 19. Iyer V, Vo Q, Mell A, Chinniah S, Zenerovitz A, Venkiteswaran K, et al. Acute levodopa dosing around-the-clock ameliorates REM sleep without atonia in hemiparkinsonian rats. NPJ Parkinsons Dis 2019;5:27.ArticlePubMedPMC
- 20. Tan A, Salgado M, Fahn S. Rapid eye movement sleep behavior disorder preceding Parkinson’s disease with therapeutic response to levodopa. Mov Disord 1996;11:214–216.ArticlePubMed
- 21. Stocchi F, Barbato L, Nordera G, Berardelli A, Ruggieri S. Sleep disorders in Parkinson’s disease. J Neurol 1998;245(Suppl 1):S15–S18.ArticlePubMed
- 22. Wailke S, Herzog J, Witt K, Deuschl G, Volkmann J. Effect of controlled-release levodopa on the microstructure of sleep in Parkinson’s disease. Eur J Neurol 2011;18:590–596.ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Comparison of sleep characteristics between Parkinson's disease with and without freezing of gait: A systematic review
Tracy Milane, Clint Hansen, Mathias Baptiste Correno, Matthias Chardon, Fabio A. Barbieri, Edoardo Bianchini, Nicolas Vuillerme
Sleep Medicine.2024; 114: 24. CrossRef - Opicapone versus entacapone: Head‐to‐head retrospective data‐based comparison of healthcare resource utilization in people with Parkinson's disease new to catechol‐O‐methyltransferase (COMT) inhibitor treatment
Glynn Harrison‐Jones, Xiaocong Li Marston, Francesca Morgante, K. Ray Chaudhuri, Guillermo Castilla‐Fernández, Valentina Di Foggia
European Journal of Neurology.2023; 30(10): 3132. CrossRef - Management of REM sleep behavior disorder: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment
Michael Howell, Alon Y. Avidan, Nancy Foldvary-Schaefer, Roneil G. Malkani, Emmanuel H. During, Joshua P. Roland, Stuart J. McCarter, Rochelle S. Zak, Gerard Carandang, Uzma Kazmi, Kannan Ramar
Journal of Clinical Sleep Medicine.2023; 19(4): 769. CrossRef - The real-life effect of catechol-O-methyltransferase inhibition on non-motor symptoms in levodopa-treated Parkinson’s disease: opicapone versus entacapone
Valentina Leta, Daniel J. van Wamelen, Federico Aureli, Vinod Metta, Dhaval Trivedi, Pietro Cortelli, Carmen Rodriguez-Blazquez, Alexandra Rizos, K. Ray Chaudhuri
Journal of Neural Transmission.2023; 130(7): 925. CrossRef - Non-oral continuous drug delivery based therapies and sleep dysfunction in Parkinson’s disease
P. Tall, M. A. Qamar, L. Batzu, V. Leta, C. Falup-Pecurariu, K. Ray Chaudhuri
Journal of Neural Transmission.2023; 130(11): 1443. CrossRef - Tenuigenin promotes non-rapid eye movement sleep via the GABAA receptor and exerts somnogenic effect in a MPTP mouse model of Parkinson's disease
Di Zhang, Wenjing Zhang, Shumin Deng, Lu Liu, Hua Wei, Fenqin Xue, Hui Yang, Xiaomin Wang, Zheng Fan
Biomedicine & Pharmacotherapy.2023; 165: 115259. CrossRef - Neurological Insights into Sleep Disorders in Parkinson’s Disease
Subramanian Thangaleela, Bhagavathi Sundaram Sivamaruthi, Periyanaina Kesika, Subramanian Mariappan, Subramanian Rashmi, Thiwanya Choeisoongnern, Phakkharawat Sittiprapaporn, Chaiyavat Chaiyasut
Brain Sciences.2023; 13(8): 1202. CrossRef - Real‐world considerations regarding the use of the combination of levodopa, carbidopa, and entacapone (Stalevo®) in Parkinson's disease
Heinz Reichmann
European Journal of Neurology.2023; 30(S2): 15. CrossRef - Clinical profile of levodopa-carbidopa-entacapone intestinal gel infusion in patients with advanced Parkinson’s disease
Karina A. Atanasova-Ivanova, Sonya Ivanova Hristova-Chakmakova, Ivan G. Milanov
Folia Medica.2023; 65(6): 929. CrossRef - The Home-Based Sleep Laboratory
Yael Hanein, Anat Mirelman, Anat Mirelman, E. Ray Dorsey, Patrik Brundin, Bastiaan R. Bloem
Journal of Parkinson's Disease.2021; 11(s1): S71. CrossRef - Shudi Pingchan Decoction combined with repetitive transcranial magnetic stimulation in the treatment of Parkinson’s disease with sleep disorders
Qing Ye, Xiqun Chen, Yuqing Hu, Jie Zhou, Chen Gao, Zhenguo Liu
Traditional Medicine and Modern Medicine.2020; 03(02): 85. CrossRef
Comments on this article
- Figure
- Related articles
-
- A Survey of Perspectives on Telemedicine for Patients With Parkinson’s Disease
- Potential Link Between Cognition and Motor Reserve in Patients With Parkinson’s Disease
- Hand Movement-Induced Eyeblink Bursts in a Patient With Parkinson’s Disease
- Umami and Other Taste Perceptions in Patients With Parkinson’s Disease
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite