Articles
- Page Path
- HOME > J Mov Disord > Volume 15(3); 2022 > Article
-
Brief communication
Movement Disorders Resulting From Bilateral Basal Ganglia Lesions in End-Stage Kidney Disease: A Systematic Review -
Kah Hui Yap1*
 , Nurul Husna Baharudin1*
, Nurul Husna Baharudin1* , Abdul Halim Abdul Gafor1
, Abdul Halim Abdul Gafor1 , Rabani Remli1
, Rabani Remli1 , Shen-Yang Lim2
, Shen-Yang Lim2 , Wan Asyraf Wan Zaidi1
, Wan Asyraf Wan Zaidi1 , Shahrul Azmin1
, Shahrul Azmin1 , Shahizon Azura Mohamed Mukari3
, Shahizon Azura Mohamed Mukari3 , Raihanah Abdul Khalid4
, Raihanah Abdul Khalid4 , Norlinah Mohamed Ibrahim1
, Norlinah Mohamed Ibrahim1
-
Journal of Movement Disorders 2022;15(3):258-263.
DOI: https://doi.org/10.14802/jmd.21185
Published online: May 26, 2022
1Department of Medicine, UKM Medical Center, Kuala Lumpur, Malaysia
2Division of Neurology, Department of Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia
3Department of Radiology, UKM Medical Center, Kuala Lumpur, Malaysia
4Department of Neurology, Assunta Hospital, Selangor, Malaysia
- Corresponding author: Norlinah Mohamed Ibrahim, MRCPI Department of Medicine, UKM Medical Center, 56000 Kuala Lumpur, Malaysia / Tel: +603-91456083/+603-91457312 / E-mail: norlinah@ppukm.ukm.edu.my
- *This authors contributed equally to this work.
Copyright © 2022 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,580 Views
- 97 Download
ABSTRACT
-
Objective
- The basal ganglia (BG) are susceptible to fluctuations in blood urea levels, sometimes resulting in movement disorders. We described patients with end-stage kidney disease (ESKD) presenting with movement disorders associated with bilateral BG lesions on imaging.
-
Methods
- We report four patients and systematically reviewed all published cases of ESKD presenting with movement disorders and bilateral BG lesions (EBSCOhost and Ovid).
-
Results
- Of the 72 patients identified, 55 (76.4%) were on regular dialysis. Parkinsonism was the most common movement disorder (n = 39; 54.2%), followed by chorea (n = 24; 33.3%). Diabetes mellitus (n = 51; 70.8%) and hypertension (n = 16; 22.2%) were the most common risk factors. Forty-three (59.7%) were of Asian ethnicity. Complete clinical resolution was reported in 17 (30.9%) patients, while 38 (69.1%) had incomplete clinical resolution with relapse. Complete radiological resolution occurred in 14 (34.1%) patients.
-
Conclusion
- Movement disorders associated with BG lesions should be recognized as a rare and potentially reversible metabolic movement disorder in patients with ESKD.
- We described four cases and performed a literature search in EBSCOhost and Ovid using Medical Subject Headings (MeSH) terms with no language restrictions (September 9, 2021): [(“renal disease” OR “uraemia”) AND (“basal ganglia” OR “striatal” OR “putaminal” OR “caudate” OR “hyperintensity” OR “hypodensity”) AND (“movement disorder” OR “chorea” OR “Parkinsonism”)]. This study was approved by the Research Ethics Committee of UKM (JEP-2021-689).
MATERIALS & METHODS
- Of the 1,033 articles screened, 38 articles fulfilled our search term criteria, yielding a total of 72 patients (Supplementary Figure 1 in the online-only Data Supplement), and details were outlined in Supplementary Table 1 (in the online-only Data Supplement). Approximately 43 (59.7%) patients were Asians. The most common premorbid/comorbid conditions were type 2 diabetes mellitus (T2DM) (n = 51; 70.8%), followed by hypertension (n = 16; 22.2%). Parkinsonism was the most common movement disorder (n = 39; 54.2%), followed by chorea (n = 24; 33.3%) (Table 1). Most patients were on regular dialysis before the onset of the movement disorder (n = 55; 76.4%). Therapy was mainly supportive involving more intensive dialysis, correction of hyperglycemia with insulin therapy, adequate hydration, and symptomatic therapy for the movement disorder.
- Neuroimaging findings included bilateral BG hypodensities on CT (n = 22), bilateral BG hypointensities on magnetic resonance imaging (MRI) T1WI with corresponding T2WI hyperintensities, and hyperintensities in the corresponding regions on diffusion-weighted imaging (DWI) and on apparent diffusion coefficient (ADC) sequences. Seven patients had positron emission tomography (PET)/single photon emission computed tomography (SPECT) imaging, which showed hypoperfusion in the BG (n = 4). Two patients’ magnetic resonance sepectroscopy (MRS) findings showed a low N-acetyl aspartate ratio (NAA) in the BG; one also showed an elevated lactate peak.
- Clinical outcome data were available for 55 patients; 17 (30.9%) had complete resolution of the movement disorder (parkinsonism: n = 7; chorea: n = 10), while 38 (69.1%) had incomplete resolution (parkinsonism: n = 25; chorea: n = 13). Complete resolution was more frequently observed with chorea than parkinsonism (43.5% vs. 21.9%; p = 0.09). Radiological follow-up was available for 41 patients, with complete resolution in 14 patients (34.1%), while 27 patients showed incomplete resolution (65.9%) (Table 1).
RESULTS
- Case 1
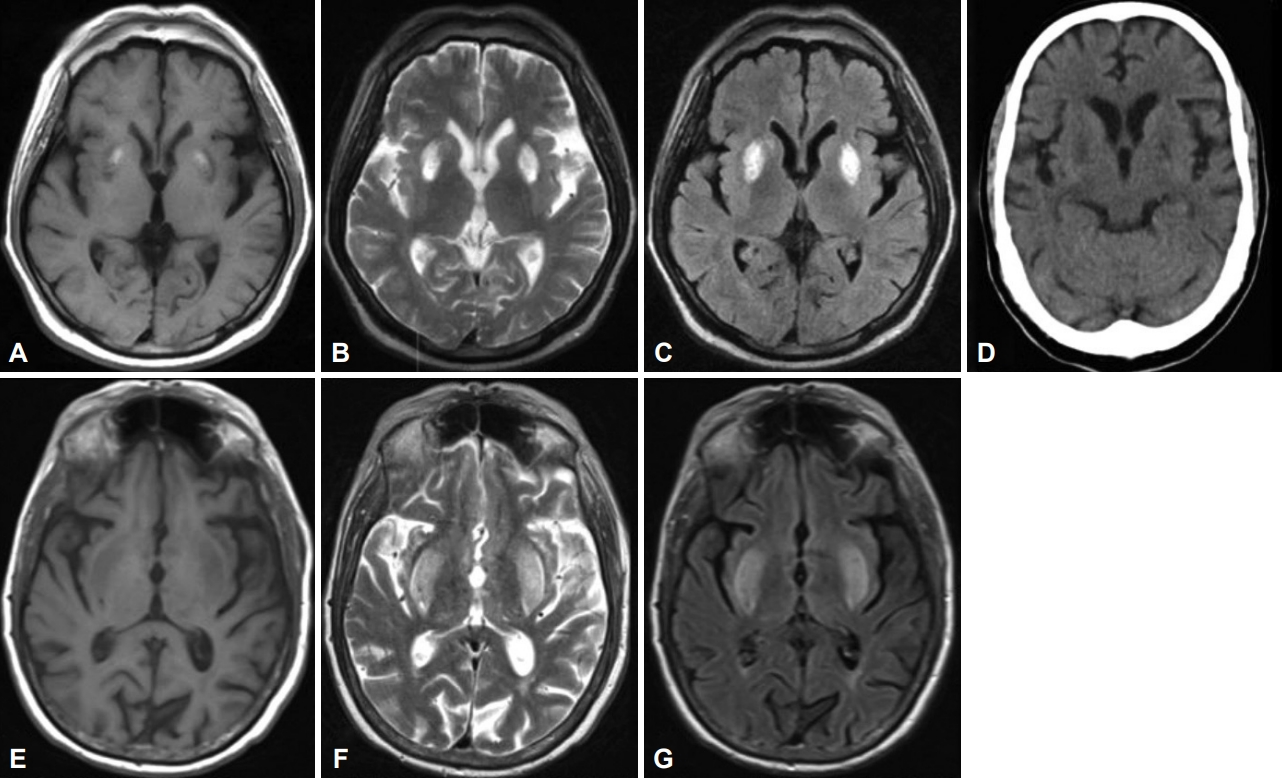
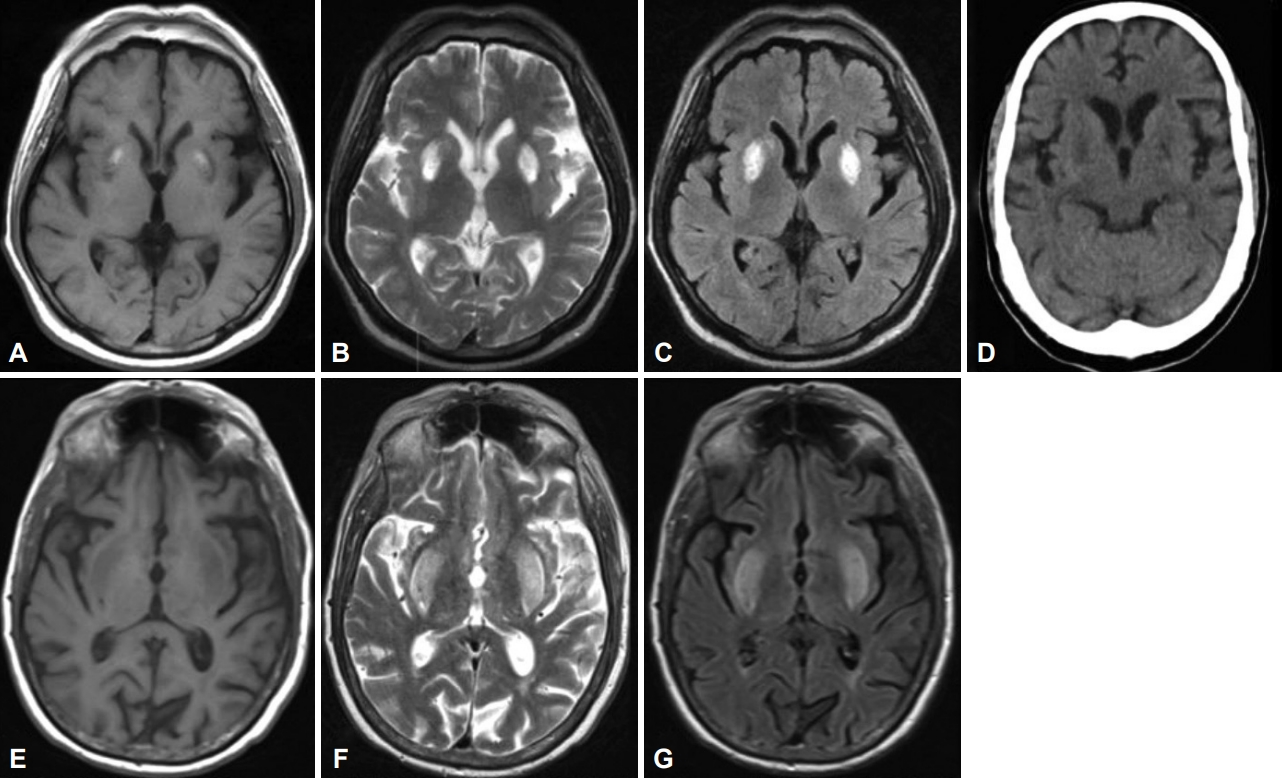
- A 49-year-old Malay man with T2DM and hypertension for 20 years and ESKD on regular hemodialysis for ten years presented with a three-month history of generalized ‘involuntary movements’ that was acute in onset. The movements worsened when he was stressed or performed specific tasks and affected his daily routine and sleep. There was no family history of similar illnesses or neurological disorders or history of hyponatremia (or rapid correction thereof). Examination revealed generalized chorea predominantly affecting his upper limbs and face, which worsened upon action and speaking. The rest of the neurological examination was normal. The Montreal Cognitive Assessment score was 23/30 (primary school education). The urea level was 11.5 mmol/L, serum creatinine level was 634 µmol/L, and HbA1C was 6.0%. Antinuclear antibody, anti-dsDNA, lupus anticoagulant and anti-cardiolipin antibodies, serum ceruloplasmin, manganese, and tumor markers (carcinoembryonic antigen, carbohydrate antigen 19-9, prostate-specific antigen, and alpha fetoprotein) were negative or within the normal range. Brain MRI showed symmetrical hyperintense lesions in the bilateral lentiform nuclei on T2WI and fluid-attenuated inversion recovery (FLAIR) (Figure 1A-C). MRA of the cerebral arteries showed right middle cerebral artery atherosclerosis (not shown). The dialysis regimen was optimized by his nephrologist, with daily dialysis and high-flux dialyzers to improve dialysis efficiency. Trials of medications including haloperidol (10 mg, bid), clonazepam (2 mg, bid), tetrabenazine (25 mg, bid), and amantadine (50 mg, bid) resulted in only minimal improvement despite multiple adjustments.
- Case 2
- A 43-year-old Malay man with long-standing T2DM, hypertension, and ESKD on regular hemodialysis for three months presented with a three-month history of generalized body weakness and progressive slowness in movement and hypophonia. He required a walking frame to ambulate at home. There was no history of recurrent falls, memory disturbances, or urinary incontinence. Physical examination revealed bilateral cogwheel rigidity, bradykinesia, and intermittent resting tremor of the left hand. His medications included amlodipine, perindopril, clopidogrel, sitagliptin, frusemide, and calcium carbonate. Unenhanced CT of the brain showed symmetrical hypodensities in the bilateral BG (Figure 1D). Blood urea and serum creatinine levels were not available as they were assessed in another center and were not available during the initial consultation. Following the initial consultation, the patient failed to return for subsequent follow-up. His family members provided a recent update that the patient had passed away two years after his initial visit due to an unrelated cause.
- Case 3
- A 68-year-old Indian man was diagnosed with T2DM, hypertension, and ESKD. He had been on regular hemodialysis since 2015 and presented in June 2018 for ‘lack of strength’ in his legs. He also complained of deterioration in the two months preceding his clinic visit, which led to the use of a wheelchair. There was no history of urinary symptoms, memory problems, or falls. On examination, there was hypomimia, jaw tremor, bradykinesia on finger taps and generalized rigidity consistent with parkinsonism. His renal profile showed elevated blood urea (18.8 mmol/L) and serum creatinine (914.8 µmol/L) levels. He was initiated on levodopa with modest improvement in his bradykinesia. Brain CT showed symmetrical hypodensities within the bilateral BG regions.
- Case 4
- A 71-year-old Indian man with hypertension and ESKD on regular hemodialysis at another center for four years presented with a three-day history of bilateral generalized choreiform movements involving the tongue, which caused difficulty ambulating and eating. He had received the Comirnaty vaccine (Pfizer-BioNTech) two weeks prior to his presentation. He also complained of fatigue, vomiting, and loss of appetite three weeks prior to presentation. On examination, there was moderate to severe generalized severe chorea, which also involved his tongue. His blood urea level was 20 mmol/L, and serum creatinine level was 1,000 µmol/L (one day after hemodialysis). Thyroid function test, serum ammonia, liver function test, diabetic screen, erythrocyte sedimentation rate, C-reactive protein, lupus screen, and anemia screen were normal or negative. Brain MRI showed bilateral lentiform hyperintensity on T2-weighted MRI and FLAIR (Figure 1E-G). He was treated with oral sulpiride and underwent another hemodialysis the same day. After dialysis, the chorea was less severe. EEG was normal. He had no further choreiform movements by the 3rd day.
Case series
- We reported four ESKD patients with acute/subacute movement disorders associated with bilateral BG lesions and systematically reviewed 72 such cases from its initial description in 1998. Parkinsonism was the most common movement disorder, followed by chorea. The radiological findings were relatively homogenous with symmetrical bilateral BG lesions on CT and MRI [3], with DWI and ADC hyperintensities indicating a possible vasogenic origin. Complete clinical resolution occurred in 30.9% of the patients, while radiological resolution occurred in only 34.1% of the patients, suggesting that this syndrome may be fully reversible, although we are unable to determine the predictors for full recovery based on this review. Recovery was associated with more intensive dialysis and appropriate management of the movement disorder. The patients presenting with chorea were more likely to fully recover than those with parkinsonism.
- The underlying pathogenesis of this syndrome remains unclear. T2DM, hypertension, and ESKD may collectively lead to metabolic acidosis, oxidative stress, and microvascular changes, increasing the risk of ischemic damage to the BG. In support of this notion, MRS findings showed reduced metabolism [4,5], and PET/SPECT scans showed hypoperfusion [6-9] within the BG. The presence of a lactate peak on MRS implied the possibility of selective mitochondrial dysfunction within the BG [10]. The abrupt onset and spontaneous recovery of the movement disorder in some patients suggested that acute ischemia may be the final insult [11]. Conversely, chronic and continual metabolic derangement in severe ESKD may lead to permanent damage to the BG structures [12], which could explain the lack of clinical improvement with dialysis in some patients. A recent review suggested manganese toxicity as a cause of BG lesions in ESKD patients with parkinsonism [13]. However, only the patient in Case 1 had manganese levels tested, which were within the normal range.
- Slightly more than half of the patients identified by this review were Asians. It is unclear whether this syndrome is more prevalent among Asians, similar to nonketotic hyperglycemia-induced chorea (NKHC), which also affects BG structures [14]. While this observation could be entirely due to higher reporting by researchers from Asian countries, it also raises the question of whether Asians are generally more predisposed toward metabolic BG insults.
- Limitations and conclusion
- There are several limitations in this review. First, clinical and radiological outcome measures were not consistently reported. Second, we were unable to determine the long-term outcome, as no prospective follow-up findings were reported in most of the reports. Nevertheless, we believe this review adds to the expanding literature on this syndrome and points toward the possibility of endogenic susceptibility among specific individuals. Future studies should investigate the long-term outcomes of this condition and determine the underlying predisposing causes.
DISCUSSION
Supplementary Materials
Supplementary Table 1.
Supplementary Figure 1.
-
Conflicts of Interest
The authors have no financial conflicts of interest.
-
Funding Statement
None
-
Author Contributions
Conceptualization: Nurul Husna Baharudin, Norlinah Mohamed Ibrahim. Data curation: Kah Hui Yap, Nurul Husna Baharudin. Formal analysis: Kah Hui Yap. Investigation: Kah Hui Yap, Nurul Husna Baharudin. Methodology: Kah Hui Yap, Nurul Husna Baharudin. Project administration: Kah Hui Yap, Nurul Husna Baharudin. Resources: Kah Hui Yap. Supervision: Norlinah Mohamed Ibrahim. Validation: Norlinah Mohamed Ibrahim. Visualisation: all authors. Writing—original draft: Kah Hui Yap, Nurul Husna Baharudin. Writing—review & editing: Abdul Halim Abdul Gafor, Rabani Remli, ShenYang Lim, Wan Asyraf Wan Zaidi, Shahrul Azmin, Shahizon Azura Mohamed Mukari, Raihanah Abdul Khalid, Norlinah Mohamed Ibrahim.
Notes
- 1. Bhagwan S, Marais S, Bhagwan B, Bhigjee AI. Reversible syndrome of extrapyramidal movement disorders with bilateral basal ganglia lesions in uremia: a case series and review of the literature. Afr J Neurol Sci 2018;37:47–51.
- 2. Hamed S, Mohamed K, Abd Elhameed S, Moussa E, Abozaid H, Lang A, et al. Movement disorders due to selective basal ganglia lesions with uremia. Can J Neurol Sci 2020;47:350–365.ArticlePubMed
- 3. Van Cauter S, Severino M, Ammendola R, Van Berkel B, Vavro H, van den Hauwe L, et al. Bilateral lesions of the basal ganglia and thalami (central grey matter)-pictorial review. Neuroradiology 2020;62:1565–1605.ArticlePubMedPMC
- 4. Dicuonzo F, Di Fede R, Salvati A, Palma M, de Mari M, Baldassarre GD, et al. Acute extrapyramidal disorder with bilateral reversible basal ganglia lesions in a diabetic uremic patient: diffusion-weighted imaging and spectroscopy findings. J Neurol Sci 2010;293:119–121.ArticlePubMed
- 5. Tajima Y, Mito Y, Yanai M, Fukazawa Y. Unusual basal ganglia lesions in a diabetic uraemic patient proven to be demyelination: first pathological observation. BMJ Case Rep 2012;2012:bcr2012006522.ArticlePubMedPMC
- 6. Choi EK, Oh JK, Chung YA, Song IU. Brain SPECT and MRI findings in a uremic patient with parkinsonism. Clin Nucl Med 2015;40:e453–e454.ArticlePubMed
- 7. Ishii K, Ishii K, Shioya A, Nemoto K, Tamaoka A. Decreased dopamine transporter and receptor ligand binding in Parkinsonism with diabetic uremic syndrome. Ann Nucl Med 2016;30:320–324.ArticlePubMed
- 8. Lee PH, Shin DH, Kim JW, Song YS, Kim HS. Parkinsonism with basal ganglia lesions in a patient with uremia: evidence of vasogenic edema. Parkinsonism Relat Disord 2006;12:93–96.ArticlePubMed
- 9. Wang HC, Hsu JL, Shen YY. Acute bilateral basal ganglia lesions in patients with diabetic uremia: an FDG-PET study. Clin Nucl Med 2004;29:475–478.ArticlePubMed
- 10. Lunsing RJ, Strating K, de Koning TJ, Sijens PE. Diagnostic value of MRS-quantified brain tissue lactate level in identifying children with mitochondrial disorders. Eur Radiol 2017;27:976–984.ArticlePubMedPMC
- 11. Robbins NM, Swanson RA. Opposing effects of glucose on stroke and reperfusion injury: acidosis, oxidative stress, and energy metabolism. Stroke 2014;45:1881–1886.ArticlePubMedPMC
- 12. Cirillo G, Cirillo M, Panetsos F, Virtuoso A, Papa M. Selective vulnerability of basal ganglia: insights into the mechanisms of bilateral striatal necrosis. J Neuropathol Exp Neurol 2019;78:123–129.ArticlePubMed
- 13. Safarpour Y, Vaziri ND, Jabbari B. Movement disorders in chronic kidney disease–a descriptive review. J Stroke Cerebrovasc Dis 2021;30:105408.ArticlePubMed
- 14. Oh Y, Lee KY, Im JH, Lee MS. Chorea assocaited with non-ketotic hyperglycemia and hyperintensity basal ganglia lesion on T1-weighted brain MRI study: a meta-analysis of 53 cases including four present cases. J Neuro Sci 2002;200:57–62.Article
REFERENCES
Figure & Data
References
Citations

Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite