Articles
- Page Path
- HOME > J Mov Disord > Volume 16(1); 2023 > Article
-
Review Article
Movement Disorders Associated With Radiotherapy and Surgical Procedures -
Bharath Kumar Surisetti1*
 , Shweta Prasad1,2*
, Shweta Prasad1,2* , Vikram Venkappayya Holla1
, Vikram Venkappayya Holla1 , Nitish Kamble1
, Nitish Kamble1 , Ravi Yadav1
, Ravi Yadav1 , Pramod Kumar Pal1
, Pramod Kumar Pal1

-
Journal of Movement Disorders 2023;16(1):42-51.
DOI: https://doi.org/10.14802/jmd.22092
Published online: January 12, 2023
1Department of Neurology, National Institute of Mental Health & Neurosciences, Bengaluru, Karnataka, India
2Department of Clinical Neurosciences, National Institute of Mental Health & Neurosciences, Bengaluru, Karnataka, India
- Corresponding author: Pramod Kumar Pal, MD, DNB, DM, FRCP Department of Neurology, National Institute of Mental Health & Neurosciences, Hosur Road, Bengaluru, Karnataka 560029, India / Tel: +91-80-26995147 / Fax: +91-80-26564830 / E-mail: palpramod@hotmail.com
- *This authors contributed equally to this work.
Copyright © 2023 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Occasionally, movement disorders can occur following interventional procedures including but not limited to radiotherapy, dental procedures, and cardiac, cerebral and spinal surgeries. The majority of these disorders tend to be unexpected sequelae with variable phenomenology and latency, and they can often be far more disabling than the primary disease for which the procedure was performed. Owing to poor knowledge and awareness of the problem, delays in diagnosing the condition are common, as are misdiagnoses as functional movement disorders. This narrative review discusses the phenomenology, pathophysiology, and potential treatments of various movement disorders caused by interventional procedures such as radiotherapy and neurological and non-neurological surgeries and procedures.
- Iatrogenic movement disorders refer to movement disorders that occur due to treatment-related adverse effects. Although iatrogenic movement disorders are most often associated with medication, i.e., tardive disorders, a wide range of interventions, either medical or surgical, have been implicated in their generation. These can include chemotherapeutic and anesthetic agents, antipsychotics, radiotherapy, and neurological and non-neurological surgeries [1-6].
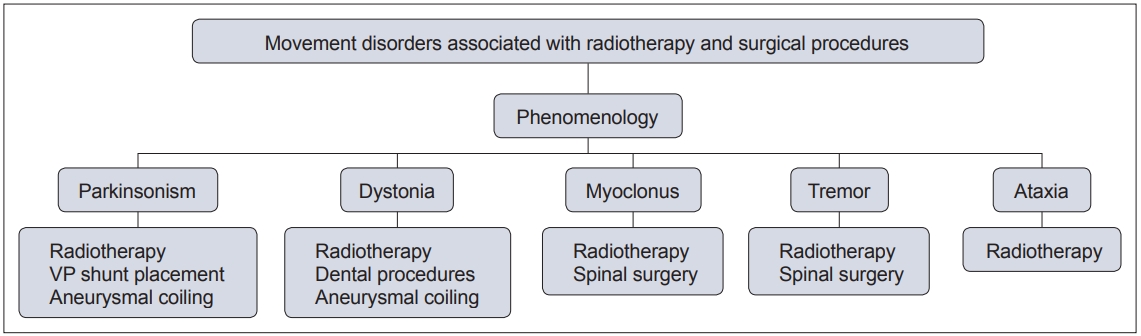
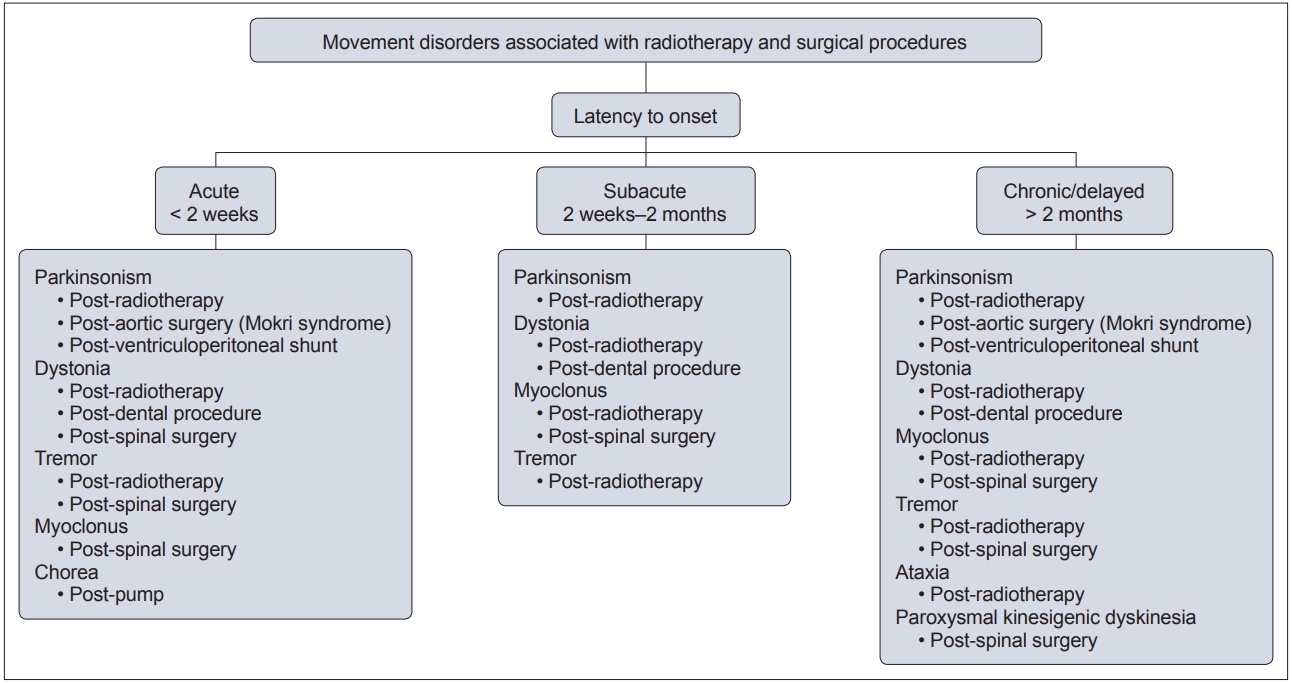
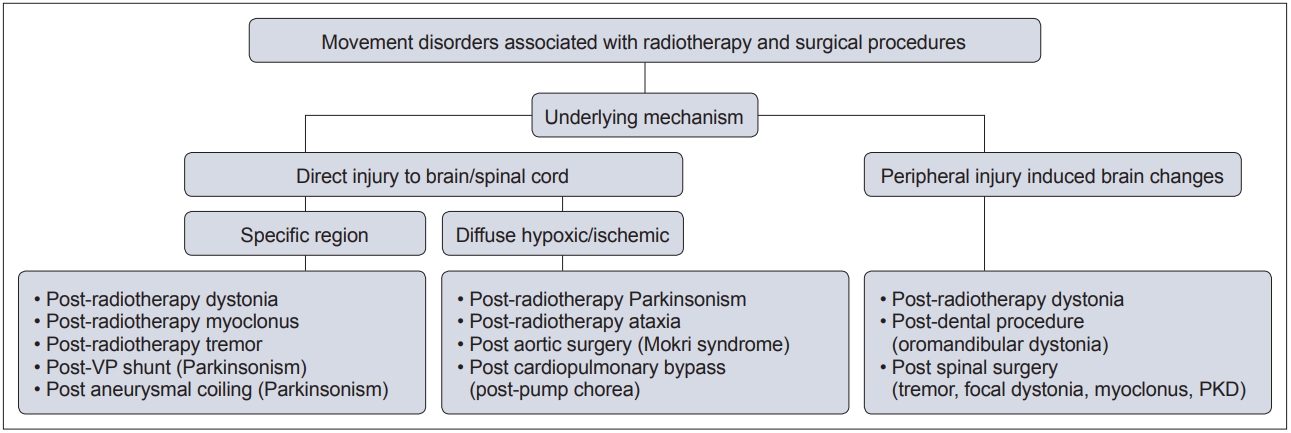
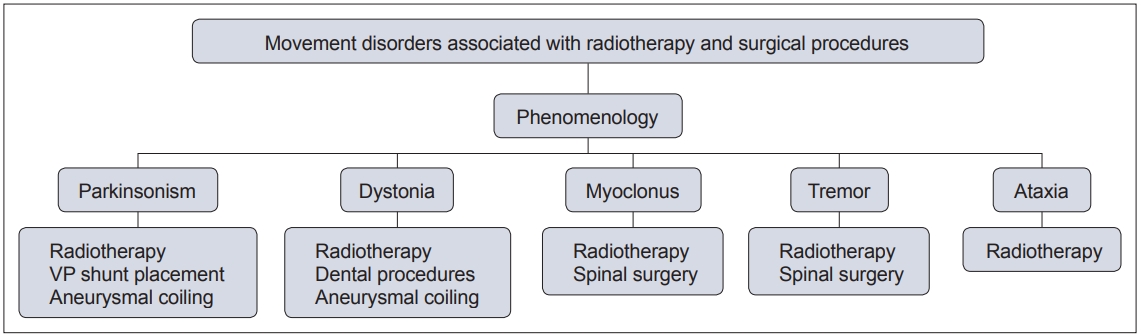
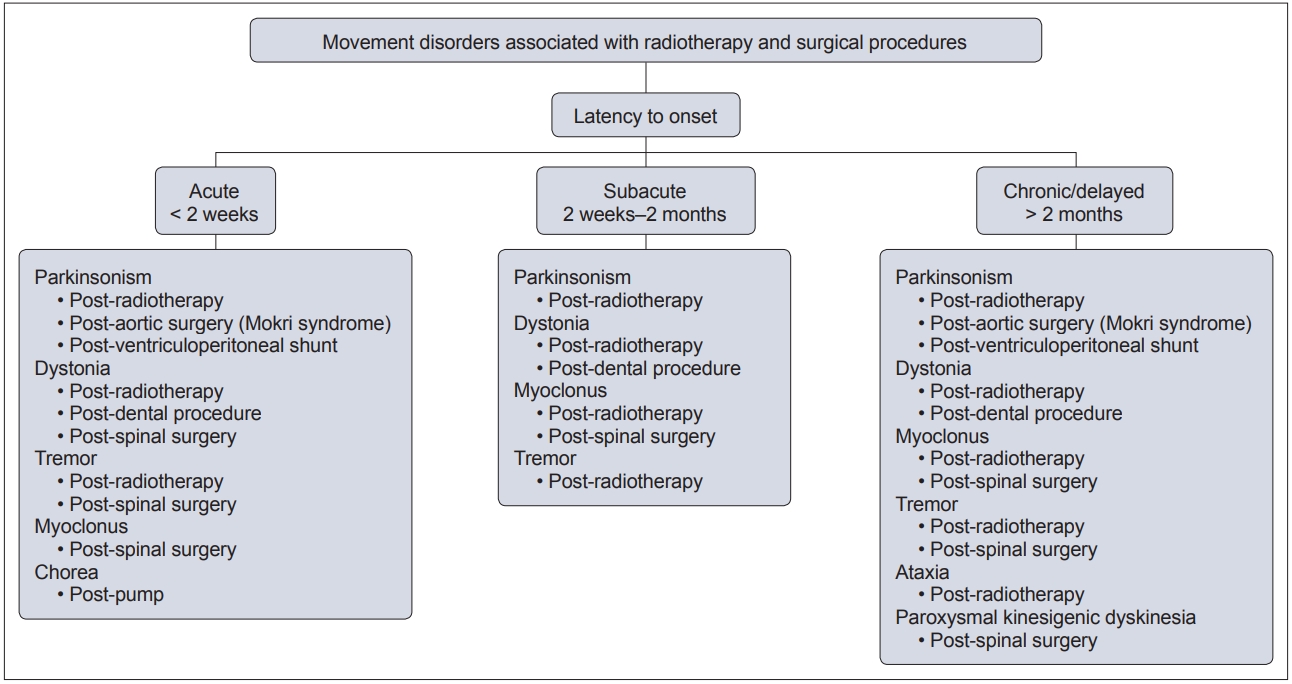
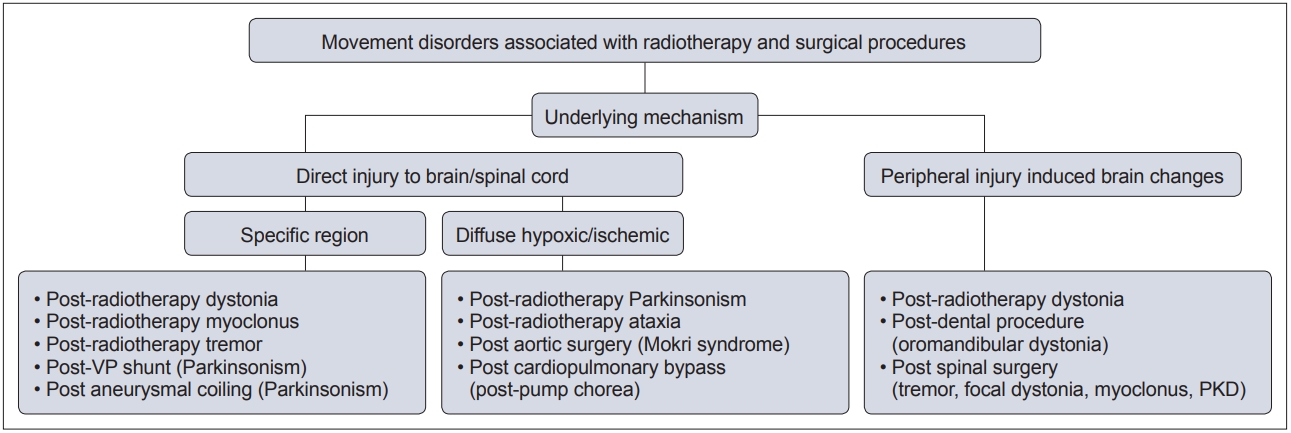
- In this context, movement disorders can occur following interventional procedures (Figure 1), including but not limited to radiotherapy [7], dental procedures [8], cardiac, cerebral and spinal surgeries [9] and deep brain stimulation (DBS) [6]. These disorders tend to be rare with an uncertain prevalence and incidence. The majority of cases are unexpected sequelae with variable phenomenology and latency, and they can often be far more disabling than the primary disease for which the procedure was performed. Owing to poor understanding and awareness of the problem, delays in diagnosing the condition are common, as is misdiagnosis as a functional movement disorder. At present, there is no established system of classification for these disorders, and the latency of onset is highly variable, even with the same underlying procedure (Figure 2). Furthermore, the underlying mechanism varies based on the causative procedure, with variability for the same phenomenology (Figure 3).
- This narrative review aims to review the phenomenology, pathophysiology, and potential treatments of various movement disorders caused by radiotherapy and neurological and non-neurological surgical procedures. DBS-related movement disorders [6] are predominantly stimulation induced and have been extensively reviewed elsewhere. They are therefore not included in the present review.
INTRODUCTION
- Radiotherapy is an important modality of treatment in various neurological diseases, such as tumors and arteriovenous malformations (AVMs). However, radiation can often cause significant neurological injury owing to damage to normal tissue. Post radiation adverse effects have been previously categorized into immediate, subacute (< 6 months) and delayed (> 6 months) [5]. While immediate and subacute complications may be reversible, delayed complications are considered to be permanent [5]. Almost all core movement disorders, i.e., parkinsonism, dystonia, tremor, myoclonus, and ataxia, have been reported post radiotherapy (Table 1). Although the exact site of injury for each of these phenomena may vary, immediate complications are suggested to be due to disruption of the blood–brain barrier by endothelial apoptosis, which leads to vasogenic edema and increased intracranial pressure. Subacute complications are proposed to occur due to demyelination, and delayed complications may occur through damage to the vasculature, i.e., loss of normal vessels or progressive fibrosis with or without neovascularization [5].
- Post radiotherapy parkinsonism
- Post radiotherapy parkinsonism can occur within a wide latency range, from between a few weeks and 39 years post radiotherapy [7], and to date, only a few cases have been reported (Table 1). Except for a single case that presented with tremordominant parkinsonism [10], all other cases presented with akinetic-rigid parkinsonism [7,11,12]. Magnetic resonance imaging (MRI) in subacute cases show predominantly globus pallidus hyperintensities, whereas in delayed onset cases, diffuse white matter hyperintensities consistent with radiation encephalopathy are evident. In addition, dopamine transporter scans show decreased uptake in the putamen [7]. The underlying pathophysiology may differ based on the time of onset of the movement disorder. In subacute postradiotherapy parkinsonism, the suggested mechanism is vasogenic edema, whereas hypoxia-induced vasculopathy is suggested for delayed (more than 2 months) post radiotherapy parkinsonism [11,12]. Treatment with levodopa is usually unsatisfactory owing to the post synaptic pathology of post radiation parkinsonism [7,12]. However, it may rarely be levodopa responsive [10,11] and reversible [11].
- Post radiotherapy dystonia
- Postradiotherapy dystonia primarily comprises cervical, oromandibular (OMD) and segmental dystonia, with occurrence close to the site of radiotherapy (Table 1) [13-15]. Similar to post radiotherapy parkinsonism, there is variable latency in the onset of post radiotherapy dystonia, which can range from 3 weeks to 5 years.
- Post radiotherapy cervical dystonia has been described in patients who received radiotherapy for laryngeal [15] and lung [14] carcinoma. In these patients, tonic muscular activity and co-contraction of the affected muscles consistent with dystonia were observed on electromyography (EMG) with the absence of neuropathic or myopathic potentials. Imaging is usually normal; however, in one case with post radiotherapy cervical dystonia, spinal MRI showed hyperintensities in the cervical spine from the C3 to C6 segments without mass effect [13]. It is imperative to differentiate dystonia from contractures wherein atrophy of the affected muscles is seen rather than hypertrophy.
- Post radiotherapy OMD has been reported following radiotherapy for nasopharyngeal carcinoma [13]. In both reported cases, patients developed jaw-closing dystonia with involvement of the masseters after a latency of 3 weeks to 3 months, and EMG showed co-contraction of the affected muscles (masseter and platysma), consistent with dystonia [13]. Post radiotherapy OMD is suggested to be a peripheral injury-related movement disorder, wherein mild but repeated injury to the trigeminal nerve due to focal radiotherapy leads to reorganization of its somatosensory representation in the thalamus. Another postulated mechanism in these cases is the involvement of the pons due to its close proximity to the radiation field. The pons has a central pattern generator function, and its involvement can lead to the generation of altered impulses to higher subcortical structures, leading to abnormal movements such as dystonia [13].
- Finally, post radiotherapy segmental dystonia involving the shoulder and trunk has been described in 3 patients who received radiotherapy for breast carcinoma [16]. These patients presented with pain and decreased range of motion of the involved region, and EMG showed involuntary motor potential firing and myokymic discharges [16].
- All forms of post radiotherapy dystonia respond poorly to anti dystonic drugs, including benzodiazepines, baclofen, and biperiden. However, they respond favorably to botulinum toxin injections, which is the drug of choice for post radiotherapy dystonia [13,16].
- Post radiotherapy myoclonus
- Post radiotherapy myoclonus has been reported to be either orthostatic [17] or spinal [18-20], with a variable latency ranging from 4 weeks to 6 years (Table 1). Post radiotherapy orthostatic myoclonus has been described in two patients who underwent wholebrain irradiation for meningioma and frontal right lobe gliosarcoma, with latency varying from subacute to chronic [17]. Both patients demonstrated high-amplitude short bursts on EMG recordings from the tibialis anterior, quadriceps and hamstrings when standing; these bursts were absent when patients were seated. Radiation-induced damage to the supplementary motor area and prefrontal cortex was suggested to contribute to the origin of myoclonus [17], which was reported to be unresponsive to levetiracetam, levodopa and clonazepam.
- Post radiotherapy spinal myoclonus has been reported in 3 cases to date, with latency periods ranging from 3 months to 6 years post-radiotherapy. In all reported cases, patients received radiotherapy that involved the spinal cord: specifically, the cervical cord [20], thoracic cord [18,20], craniospinal cord and whole cord [19]. Myoclonus was observed in areas surrounding the sites of radiotherapy. The severity was variable, with the myoclonus severe enough to lead to an acetabular fracture in one case [20]. In another case, due to a long latency of 6 years, a functional etiology was initially considered. The possibility of postradiotherapy myoclonus was only considered following the absence of Bereitschafts potential associated with the movements [19]. Imaging was reported to be normal in all reported cases, and no specific basis for myoclonus was reported. However, based on the improvement with GABAergic drugs in 2 of the cases, the possibility of underlying post radiation hyperexcitability may be considered [18]. Intrathecal morphine may be beneficial in cases of unresponsiveness to clonazepam, dantrolene or valproate [20].
- Post radiotherapy tremor
- Post radiotherapy tremor can occur either as a palatal tremor or Holmes tremor, following radiotherapy directed toward the pons and midbrain via gamma knife radiosurgery (GKRS) for AVMs [21,22] or after whole-brain irradiation [23].
- In contrast to other post radiotherapy movement disorders, post radiotherapy tremor, either palatal or Holmes, has a relatively shorter range of latency (2 weeks–3.5 months). Both reported cases of post radiotherapy palatal tremor were found to have hypertrophic olivary degeneration [21]. A variable imaging observation was reported in the post radiotherapy Holmes tremor. While a hyperintense lesion of the red nucleus was observed in the case that underwent GKRS for the AVM [22], no obvious lesion of the red nucleus was observed following whole-brain irradiation [23]. In the latter, it is possible that following irradiation, contraction of the hamartoma led to a distortion of the mesencephalon, which in turn produced a Holmes tremor [23]. No specific treatment was reported for the palatal tremor, whereas the post-GKRS Holmes tremor responded well to amantadine and trihexyphenidyl, and the post-whole-brain irradiation Holmes tremor completely resolved following surgical resection of the hamartoma.
- Post radiotherapy ataxia
- Postradiotherapy ataxia is predominantly reported following whole-brain irradiation for metastatic cancer [24-28]. The latency can range from 5 months to several years post-radiotherapy. Patients may also present with progressive dementia, ataxia, and urinary incontinence, which may resemble normal pressure hydrocephalus [28]. Radiation-induced leukoencephalopathy is considered to contribute to ataxia, and no specific treatment has been recommended, with corticosteroids and ventriculoperitoneal shunting offering incomplete improvement [28].
POST RADIOTHERAPY MOVEMENT DISORDERS
- Non neurological procedures
- Oromandibular dystonia (OMD) is a well-reported form of post-dental procedure movement disorder, and a vast variety of procedures have been implicated. These include dental extraction [29-33], ill-fitting dentures [33-35], oral surgery [36], dental implants [37], root canal treatment [33], gingivectomy [33], apicoectomy, ostectomy, TMJ arthroscopic surgery and occlusal alteration (Table 2) [38-41]. A higher prevalence has been reported among women between 40 and 70 years of age, with post procedural latency ranging from a few hours to a few years. OMD may progress to laryngospasm [34], or involvement of the tongue and neck may result in segmental distribution, rarely becoming generalized dystonia [42]. The basis for post-dental procedure OMD may be similar to that of peripheral trauma-induced dystonia [31], wherein peripheral trauma can lead to an altered somatotropic organization in the thalamus with subsequent changes in subcortical circuits, which leads to altered transmission within the basal ganglia. A few patients report transient improvement with sensory tricks, and botulinum toxin injections provide satisfactory improvement. Some patients have a history of bruxism predating the OMD; thus, it is uncertain whether such patients have a predisposition to developing OMD following the dental procedure. Facial dyskinesias (jaw opening, closing spasms, blepharospasm) have also been reported following dental procedures, with latency periods of a few days to a year following the procedure [31].
- Progressive supranuclear palsy (PSP)-like syndrome, i.e., Mokri syndrome, was first described in 2004 [43]. Occurring after aortic surgery, this relatively uncommon syndrome is characterized by a triad of supranuclear gaze palsy, dysarthria and gaze disturbance (Table 3) [43-56]. Although it is suggested that there is a higher prevalence of this syndrome in men, this is likely due to the higher prevalence of thoracic aortic aneurysms and abdominal aortic dissections in men.
- The disease is biphasic, with immediate and latent phases. In the immediate phase, supranuclear gaze palsy with mild gait disturbance, dysarthria and tremulousness can be seen. This initial phase improves or stabilizes within a few weeks. Subsequently, after a latency of a few months, progressive supranuclear down-gaze palsy with ataxic gait, dysarthria and dysphagia can develop [43]. Impairment of horizontal gaze has been more commonly reported than vertical gaze impairment [43].
- One possible explanation postulated for Mokri syndrome is the use of profound hypothermia (12.5°C to 30°C) during aortic surgeries in comparison to other cardiac surgeries [44]. Although hypothermia is considered a neuroprotective strategy, patients may develop ischemia if rewarming is carried out rapidly. Furthermore, this ischemia may further impact patients who develop hyperglycemia during hypothermia. Additionally, improper acid-base management can lead to cerebral edema. All these factors can lead to vasoconstriction and impaired microcirculation [44]. Another proposed mechanism is multiple emboli in the posterior circulation; however, structural imaging has been found to be normal in most cases [47]. While AV-1451 tau PET was normal in one case, fluorodeoxyglucose-positron emission tomography (FDG-PET) demonstrated the ‘pimple sign,’ which is consistent with PSP; however, the DAT scan was normal [44]. Mokri syndrome is unresponsive to levodopa and has a poor prognosis.
- Post-pump chorea refers to choreiform movements that occur in children who undergo major cardiac surgery needing cardiopulmonary bypass and deep hypothermia circulatory arrest (DHCA) [57]. This entity was first described in 1960 [58], and the incidence of post-pump chorea varies between 1–18 [59]. This is a rare entity, as evidenced by a recent study by Ahn et al. [60], who identified 2 patients with adult-onset post-pump chorea out of 5,338 adults who underwent cardiopulmonary bypass. Although more common in children, it may be seen in adults following pulmonary endarterectomy, aortic arch and valve replacement surgeries [61]. Chorea is usually generalized and occurs after a latency of one day to a week post-surgery. Apart from generalized chorea, orofacial dyskinesia, dysphagia, loss of tone and dysarthria can occur [62]. Risk factors can be young age (children), prolonged duration of cardiopulmonary bypass, longer duration of total circulatory arrest, hypothermia, and duration of aortic clamping [60,63].
- The proposed mechanism is cerebral vasoconstriction due to hypocapnia and alkalosis during rewarming and increased blood viscosity. Hypothermia can also result in marked cerebral vasoconstriction leading to ischemic insult. The globus pallidus interna (GPi) and striatum are vulnerable to hypoxic damage, which results in abnormal movements. MRI shows bilateral basal ganglia hyperintensities in the caudate, putamen and globus pallidus. FDG-PET indicates hypometabolism in the bilateral basal ganglia [64]. The course of this condition is variable. It has been transient in some patients and subsided after treatment with neuroleptics and dopamine blockers. In other cases, it has been severe and irreversible, with cognitive deficits and disability [65,66]. GPi DBS with good outcome has been reported in a case with disabling post-pump chorea [67]. No differences in age, sex or surgical findings were observed in adults with post-pump chorea who had a good outcome versus those who had a bad outcome. However, initial MRI signal changes in the caudate and putamen have been associated with a poor outcome [60].
- Neurological procedures
- Post-ventriculoperitoneal shunt parkinsonism usually occurs secondary to shunt malfunction rather than the procedure itself. It is more commonly observed in patients with obstructive hydrocephalus secondary to aqueductal stenosis [68]. The latency can be from 3 days to 24 years after the initial shunt placement and often follows repeated episodes of shunt failure (Table 4) [69-75]. In the case of shunt malfunction, hydrocephalus leads to pressure over the basal ganglia circuits, leading to decreased dopaminergic output, which can lead to the development of acute parkinsonian symptoms [70]. Specifically, for obstructive hydrocephalus, both bottom-up and top-down damage from the pressure gradient between the infratentorial and supratentorial space is suggested to contribute to parkinsonism [68]. The common manifestations are symmetrical parkinsonism with bradykinesia, rigidity and hypophonia. However, parkinsonism may also be asymmetrical [68]. Tremor is uncommon but has been previously reported [69].
- Decreased cerebral blood flow to the basal ganglia was observed in cases who underwent single-photon emission computerized tomography (SPECT) [70]. In a few cases with hydrocephalus, simple revision of the shunt abated the symptoms of parkinsonism, suggesting a component of reversible compression of basal ganglia. In cases of obstructive hydrocephalus, endoscopic third ventriculostomy might be beneficial [68]. Repeated episodes of shunt blockage may induce irreversible damage that may not improve with shunt surgery alone and may need levodopa therapy. This condition is levodopa responsive, and the prognosis is usually good. In a few cases, eventual withdrawal of levodopa has been possible [68,70,76].
- Similar to parkinsonism that develops following ventriculoperitoneal shunting, parkinsonism has been associated with aneurysmal coiling, although it is more likely due to mechanical compression of structures by the aneurysm or cyst that developed post-coiling rather than a direct complication of coiling. To date, two cases of hemiparkinsonism subsequent to aneurysm coiling have been described [77,78]. In the first case, hemiparkinsonism developed four weeks following coiling of a posterior cerebral artery aneurysm, and compression of the midbrain by the aneurysm sac was implicated in the genesis of parkinsonism [77]. In the other case, hemiparkinsonism developed 15 months post-coiling of an anterior cerebral artery aneurysm, and the compression of the ventrolateral cerebral peduncle by the aneurysmal sac was implicated for parkinsonism [78]. In both cases, the parkinsonism was responsive to trihexyphenidyl [77] and levodopa-carbidopa [78].
- Post-spinal surgery-associated movement disorders are a type of peripherally induced movement disorder that occur in close anatomical relationship to the operated segment. Most patients may experience pain before the onset of symptoms. Occurring more frequently after surgery of the cervical cord than other segments, a wide range of movement disorders can be observed, including tremor, focal dystonia, paroxysmal kinesigenic dystonia, and spinal myoclonus (Table 5) [9,79,80]. The latency period is also highly variable and may range from days to years post-surgery. As with post-dental procedure OMD, peripheral injury-induced changes have been implicated in the pathogenesis of post-spinal surgery movement disorders. Clonazepam has been reported to be variably beneficial. However, botulinum toxin injections may prove to be useful in focal conditions.
POST-SURGICAL MOVEMENT DISORDERS
Dental procedures
Cardiothoracic surgeries
Aortic surgery
Cardiopulmonary bypass
Ventriculoperitoneal shunt
Aneurysmal coiling
Spinal surgery
- As radiotherapy- and surgical procedure-related movement disorders are a diagnosis that is presumed based on an antecedent relationship after the procedure, it is necessary to understand the limitations of an inaccurate diagnosis. First, the frequency of occurrence is rare, various factors (radiation intensity, period, etc.) exist within one type of procedure, and the spectrum of the latency period from procedure to occurrence is wide. A few disorders, such as post-pump chorea, are transient; however, the majority are progressive with unsatisfactory response to medication. Awareness of this entity by the treating physicians and proper pre procedure neurological evaluation and counseling is necessary to ensure identification of new-onset, post procedure movement disorders.
CONCLUSIONS
-
Conflicts of Interest
The authors have no financial conflicts of interest.
-
Funding Statement
None.
-
Author Contributions
Conceptualization: Pramod Kumar Pal. Data curation: Bharath Kumar Surisetti, Shweta Prasad. Formal analysis: Bharath Kumar Surisetti, Shweta Prasad. Investigation: Bharath Kumar Surisetti, Shweta Prasad. Methodology: Pramod Kumar Pal. Supervision: Vikram Venkappayya Holla, Nitish Kamble, Ravi Yadav, Pramod Kumar Pal. Validation: Vikram Venkappayya Holla, Nitish Kamble, Ravi Yadav, Pramod Kumar Pal. Visualization: Bharath Kumar Surisetti, Shweta Prasad. Writing—original draft: Bharath Kumar Surisetti, Shweta Prasad. Writing—review & editing: Vikram Venkappayya Holla, Nitish Kamble, Ravi Yadav, Pramod Kumar Pal.
Notes
| Reference | Phenomenology | No. | Latency | Associated cancer | Treatment | |
|---|---|---|---|---|---|---|
| Parkinsonism | ||||||
| Mehanna et al. [7], 2016 | Akinetic rigid | 3 | Immediate | Glioblastoma | Levodopa resistant | |
| 7 years | Temporal glioma | Levodopa resistant | ||||
| 39 years | Posterior fossa tumor | Levodopa resistant | ||||
| Bernard et al. [11], 2011 | Akinetic rigid | 1 | 4 weeks | Thalamic and midbrain dysgerminoma | Levodopa responsive | |
| Voermans et al. [12], 2006 | Akinetic rigid | 1 | 6 months | Craniopharyngoma | Levodopa resistant | |
| Skiming et al. [10], 2003 | Tremor dominant | 1 | 6 months | Levodopa responsive | Levodopa responsive | |
| Dystonia | ||||||
| Astudillo et al. [15], 2003 | Cervical | 1 | 3 months | Laryngeal carcinoma | NA | |
| Landan et al. [14], 1987 | Cervical | 1 | 5 years | Lung carcinoma | NA | |
| Salazar et al. [13], 2014 | Oro-mandibular | 2 | 3 weeks | Nasopharyngeal carcinoma | Botulinum toxin injection | |
| 3 months | Nasopharyngeal carcinoma | Clonazepam | ||||
| Soumekh et al. [16], 2005 | Segmental | 1 | 3 months–5 years | Breast carcinoma | Botulinum toxin injection | |
| Myoclonus | ||||||
| Ahn et al. [20], 2017 | Spinal | 1 | 1 year | Hodgkins lymphoma | Morphine | |
| Löscher et al. [19], 2003 | Spinal | 1 | 6 years | Medulloblastoma (cerebellar) | Clonazepam | |
| Askenasy et al. [18], 1988 | Spinal | 1 | 3 months | Seminoma | Carbamazepine | |
| Cutsforth-Gregory et al. [17], 2017 | Orthostatic | 2 | 4 weeks | Meningioma | No benefit with levetiracetam, clonazepam, levodopa | |
| NA | Gliosarcoma | |||||
| Tremor | ||||||
| Yun et al. [21], 2013* | Palatal | 2 | 3, 6 months | Mid brain-pontine AVM | No specific treatment given | |
| Chiou et al. [22], 2006 | Holmes | 1 | 2 weeks | Thalamic AVM | Amantadine and trihexyphenidyl | |
| Pomeranz et al. [23], 1990 | Holmes | 1 | 3 weeks | Pineal hamartoma | Surgical resection | |
| Ataxia | ||||||
| Kumar et al. [25], 2016* | Cerebellar ataxia | 11 | 34 months | Metastatic cancer | No specific treatment given | |
| Renard et al. [26], 2010 | Cerebellar ataxia | 1 | 5 months | Metastatic cancer | No specific treatment given | |
| Reference | No. of patients | Latency | Dental etiology |
|---|---|---|---|
| Chung et al. [37], 2013 | 1 | 1 year | Dental implant |
| Jang et al. [29], 2012 | 2 | NA | Dental extraction |
| Chidiac et al. [38], 2011 | 1 | NA | Occlusal adjustment |
| Thorburn et al. [30], 2009 | 2 | 3 weeks–6 months | Dental extraction |
| Balasubramaniam et al. [36], 2008 | 1 | NA | Oral surgery |
| Seeman et al. [39], 2008 | 1 | 8 weeks | Dental filling |
| Yoshida [40], 2006 | 2 | 4–8 years | Occlusional splint |
| Hamzei et al. [34], 2003 | 1 | Facial -3 hours | Ill-fitting denture |
| Laryngeal -3 days | |||
| Peñarrocha et al. [41], 2001 | 1 | 2 years | Loss of teeth and occlusal alteration |
| Schrag et al. [31]v 1999 | 8 | Hours to 1 year | Dental extraction, dental filling, dentures insertion |
| Sankhla et al. [33], 1998 | 21 | 1–16 years | Ill-fitting denture, root canal, gingivectomy, tooth removal |
| Thompson et al. [32], 1986 | 1 | NA | Dental extraction |
| Sutcher et al. [35], 1971 | 4 | 1 to many years | Ill-fitting denture |
| Reference | Procedure | No. | Symptoms and signs | Imaging | Prognosis |
|---|---|---|---|---|---|
| Tisel et al. [44], 2020 | Asc. AA, AVR, AD, AVR with bypass | 25 | SNGP, Gait impairment, dysarthria, dysphagia, PI, SZ | Midbrain atrophy, SVD, CI in basal ganglia, microbleeds or normal | Progressed in majority |
| Lee et al. [45], 2017 | Traumatic AD | 1 | SNGP, dysarthria | Multiple micro bleeds | Progressive |
| Kim et al. [48], 2014 | Replacement of thoracic aorta | 1 | SNGP, dysarthria, dysphagia | Multiple micro bleeds | Progressive |
| Nandipati et al. [47], 2013 | Asc. AA, AD | 2 | SNGP, dysarthria, dysphagia, blepharospasm, gait disturbance | Callosal and frontal hyperintensities, occipital infarction | One patient died after 2 years. Status of other patient unknown |
| Kim et al. [46], 2010 | AD | 1 | SNGP, dysarthria, dysphagia, PI | Normal | Progressive |
| Vaughan et al. [49], 2008 | Asc. AA | 1 | SNGP, dysarthria, dysphagia, PI, SZ, cognitive changes | NSWMD | Initial improvement with persistent disability |
| Eggers et al. [50], 2008 | AVR, AD | 3 | SNGP, dysarthria, seizures | SVD, hippocampal atrophy, dorsal pontine lesion | Persistent |
| Solomon et al. [51], 2008 | AD, AVR | 10 | SNGP, dysarthria, dysphagia, gait disturbances, PI, emotional change | Normal, SVD, diffuse atrophy, posterior thalamic and medial temporal high signal | NA |
| Yee et al. [52], 2007 | Asc. AA, AVR, AD, AVR with bypass | 3 | SNGP, dysarthria, dysphagia, gait disturbance, cognitive change | Normal, small infarct in cerebellum, infarct in pons, motor cortex | NA |
| Antonio-Santos et al. [53], 2007 | AAA | 1 | SNGP, Gait impairment, dysphagia | CI in parietal lobe | NA |
| Kim et al. [54], 2005 | Thoracic AA | 1 | SNGP, dysarthria, PI, dysarthria, blepharospasm, gait abnormality, emotional change | Destruction of bilateral putamen, GP, caudate | NA |
| Bernat et al. [55], 2004 | Asc.AA, AVR, AD | 2 | SNGP, Gait impairment, dysphagia, dysarthria | Small infarct in centrum semiovale, normal | One patient stabilized, the other progressed |
| Mokri et al. [43], 2004 | Asc.AD, AVR, AD | 7 | SNGP, Gait impairment, dysphagia, PI, dysarthria | Normal, CI in caudate, cerebrum, T2 hyperintensity in temporal lobe | Progressive |
| Tomsak et al. [56], 2002 | PDA, AVR | 2 | SNGP, dysarthria, emotional change, blepharospasm | NA | Stabilized |
No., number of patients; AA, aortic aneurysm; Asc., ascending; AVR, aortic valve replacement; AD, aortic dissection; SNGP, supranuclear gaze palsy; PI, postural instability; SZ, seizure; SVD, small vessel disease; CI, chronic infarct; NSWMD, nonspecific white matter disease; AAA, ascending aorta aneurysm; NA, not available; GP, globus pallidus; PDA, patent ductus arteriosus.
| Reference | No. | Latency | Previous shunts | Response to shunting | Additional treatment | Drug withdrawal at follow-up |
|---|---|---|---|---|---|---|
| Prashantha et al. [69], 2008 | 1 | 3 days | 1 | No immediate improvement | Levodopa, THP | Yes |
| Zeidler et al. [70], 1998 | 1 | 2 years | 3 | No immediate improvement | Bromocriptine, levodopa | No |
| Curran et al. [71], 1994 | 2 | 1 year | 2 | Slow improvement | Levodopa | No |
| 17 years | 1 | No improvement | Levodopa | Unknown | ||
| Gatto et al. [72], 1990 | 1 | 24 years | 1 | Disappeared after shunt | Not given | Not applicable |
| Shahar et al. [73], 1988 | 1 | 1 year | Many | No improvement | Unknown | Unknown |
| Berger et al. [74], 1985 | 1 | 9 months | 3 | Slow improvement | Benztropine, levodopa | Unknown |
| Brazin et al. [75], 1985 | 1 | 2 months | 1 | Slow improvement | Levodopa | Yes |
| Reference | Movement disorders | No. | Latency | Spinal disease | Treatment | Improvement |
|---|---|---|---|---|---|---|
| Pande et al. [79], 2020 | Spinal myoclonus | 1 | 1 month | Lumbar epidural abscess | Clonazepam | Yes |
| Sardana et al. [80], 2019 | Propriospinal myoclonus | 1 | 2 years | Dorsal spine surgery | Clonazepam and baclofen | No |
| Capelle et al. [9], 2004 | PKD | 6 | 3 months | Cervical disc herniation | Baclofen and tetrazepam | No |
| Tremor, myoclonus | 1 day | Lumbar disc herniation | Gabapentin | No | ||
| Focal dystonia | 1 week | Lumbar disc herniation | NA | No | ||
| Focal hand tremor | 3 months | Cervical disc herniation | Beta blocker, amantadine, levodopa | No | ||
| Tremor of both hands | 1 week | Cervical disc herniation | Levodopa, dopamine agonist | No | ||
| PKD | 12 months | Multiple disc herniation | NA | No |
- 1. Sposato LA, Fustinoni O. Iatrogenic neurology. Handb Clin Neurol 2014;121:1635–1671.ArticlePubMed
- 2. Stone JB, DeAngelis LM. Cancer-treatment-induced neurotoxicity--focus on newer treatments. Nat Rev Clin Oncol 2016;13:92–105.ArticlePubMedPDF
- 3. Rabinstein AA, Keegan MT. Neurologic complications of anesthesia: a practical approach. Neurol Clin Pract 2013;3:295–304.ArticlePubMedPMC
- 4. Friedman JH. Tardive syndromes. Continuum (Minneap Minn) 2019;25:1081–1098.ArticlePubMed
- 5. Nolan CP, DeAngelis LM. Neurologic complications of chemotherapy and radiation therapy. Continuum (Minneap Minn) 2015;21(2 Neurooncology):429–451.ArticlePubMed
- 6. Baizabal-Carvallo JF, Jankovic J. Movement disorders induced by deep brain stimulation. Parkinsonism Relat Disord 2016;25:1–9.ArticlePubMed
- 7. Mehanna R, Jimenez-Shahed J, Itin I. Three cases of levodopa-resistant parkinsonism after radiation therapy. Am J Case Rep 2016;17:916–920.ArticlePubMedPMC
- 8. Raoofi S, Khorshidi H, Najafi M. Etiology, diagnosis and management of oromandibular dystonia: an update for stomatologists. J Dent (Shiraz) 2017;18:73–81.PubMedPMC
- 9. Capelle HH, Wöhrle JC, Weigel R, Bäzner H, Grips E, Krauss JK. Movement disorders after intervertebral disc surgery: coincidence or causal relationship? Mov Disord 2004;19:1202–1208.ArticlePubMed
- 10. Skiming JA, McDowell HP, Wright N, May P. Secondary parkinsonism: an unusual late complication of craniospinal radiotherapy given to a 16-month child. Med Pediatr Oncol 2003;40:132–134.ArticlePubMed
- 11. Bernard G, Chouinard S. A unique pediatric case of radiation-induced parkinsonism. J Pediatric Neurol 2011;9:123–126.
- 12. Voermans NC, Bloem BR, Janssens G, Vogel WV, Sie LT. Secondary parkinsonism in childhood: a rare complication after radiotherapy. Pediatr Neurol 2006;34:495–498.ArticlePubMed
- 13. Salazar G, Español G, Fragoso M. Oromandibular dystonia secondary to radiation therapy: a description of 2 cases. Neurologia 2014;29:189–191.ArticlePubMed
- 14. Landan I, Cullis PA. Torticollis following radiation therapy. Mov Disord 1987;2:317–319.ArticlePubMed
- 15. Astudillo L, Hollington L, Game X, Benyoucef A, Boladeras AM, Delisle MB, et al. Cervical dystonia mimicking dropped-head syndrome after radiotherapy for laryngeal carcinoma. Clin Neurol Neurosurg 2003;106:41–43.ArticlePubMed
- 16. Soumekh F, Wasan A, Ross E, Michna E, Janfaza D. Effects of botulinum toxin on segmental dystonia after radiotherapy for breast cancer. J Pain 2005;6:S36.Article
- 17. Cutsforth-Gregory JK, Hammack JE, Matsumoto JY. Orthostatic myoclonus after brain tumor radiation: insights from two lesional cases. Parkinsonism Relat Disord 2017;41:109–112.ArticlePubMed
- 18. Askenasy JJ, Brunet P, Leger JM, Bouche P, Lafont F, Cathala HP, et al. Postradiation segmental myoclonus selectively inhibited by REM sleep (sleep-wake myoclonus). Eur Neurol 1988;28:317–320.ArticlePubMedPDF
- 19. Löscher WN, Trinka E. Late delayed postradiation spinal myoclonus or psychogenic movement disorder? Mov Disord 2003;18:346–349.ArticlePubMedPDF
- 20. Ahn JE, Yoo D, Jung KY, Kim JM, Jeon B, Lee MC. Spinal myoclonus responding to continuous intrathecal morphine pump. J Mov Disord 2017;10:158–160.ArticlePubMedPMCPDF
- 21. Yun JH, Ahn JS, Park JC, Kwon DH, Kwun BD, Kim CJ. Hypertrophic olivary degeneration following surgical resection or gamma knife radiosurgery of brainstem cavernous malformations: an 11-case series and a review of literature. Acta Neurochir (Wien) 2013;155:469–476.ArticlePubMedPDF
- 22. Chiou TS, Tsai CH, Lee YH. Unilateral Holmes tremor and focal dystonia after gamma knife surgery. J Neurosurg 2006;105 Suppl:235–237.ArticlePubMed
- 23. Pomeranz S, Shalit M, Sherman Y. “Rubral” tremor following radiation of a pineal region vascular hamartoma. Acta Neurochir (Wien) 1990;103:79–81.ArticlePubMedPDF
- 24. Aktan M, Koc M, Kanyilmaz G, Tezcan Y. Outcomes of reirradiation in the treatment of patients with multiple brain metastases of solid tumors: a retrospective analysis. Ann Transl Med 2015;3:325.PubMedPMC
- 25. Kumar V, Vincent D, Butler JS, Xu Y. Ataxia in long term survivors of lung cancer after whole brain radiation therapy (WBRT). J Clin Oncol 2016;34(15_suppl):e20656. Article
- 26. Renard D, Collombier L, Castelnovo G, Fourcade G, Debrigode C, Labauge P. Radiation therapy-related ataxia associated with FDG-PET cerebellar hypometabolism. Acta Neurol Belg 2010;110:100–102.PubMed
- 27. Ozgen Z, Atasoy BM, Kefeli AU, Seker A, Dane F, Abacioglu U. The benefit of whole brain reirradiation in patients with multiple brain metastases. Radiat Oncol 2013;8:186.ArticlePubMedPMCPDF
- 28. DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology 1989;39:789–796.ArticlePubMed
- 29. Jang SM, Cho YC, Sung IY, Kim SY, Son JH. Oromandibular dystonia after dental treatments: a report of two cases. J Korean Assoc Oral Maxillofac Surg 2012;38:379–383.Article
- 30. Thorburn DN, Lee KH. Oromandibular dystonia following dental treatment: case reports and discussion. N Z Dent J 2009;105:18–21.PubMed
- 31. Schrag A, Bhatia KP, Quinn NP, Marsden CD. Atypical and typical cranial dystonia following dental procedures. Mov Disord 1999;14:492–496.ArticlePubMed
- 32. Thompson PD, Obeso JA, Delgado G, Gallego J, Marsden CD. Focal dystonia of the jaw and the differential diagnosis of unilateral jaw and masticatory spasm. J Neurol Neurosurg Psychiatry 1986;49:651–656.ArticlePubMedPMC
- 33. Sankhla C, Lai EC, Jankovic J. Peripherally induced oromandibular dystonia. J Neurol Neurosurg Psychiatry 1998;65:722–728.ArticlePubMedPMC
- 34. Hamzei F, Rijntjes M, Gbadamosi J, Fuchs K, Weiller C, Münchau A. Life-threatening respiratory failure due to cranial dystonia after dental procedure in a patient with multiple system atrophy. Mov Disord 2003;18:959–961.ArticlePubMed
- 35. Sutcher HD, Underwood RB, Beatty RA, Sugar O. Orofacial dyskinesia. A dental dimension. JAMA 1971;216:1459–1463.ArticlePubMed
- 36. Balasubramaniam R, Rasmussen J, Carlson LW, Van Sickels JE, Okeson JP. Oromandibular dystonia revisited: a review and a unique case. J Oral Maxillofac Surg 2008;66:379–386.ArticlePubMed
- 37. Chung SJ, Hong JY, Lee JE, Lee PH, Sohn YH. Dental implants-induced task-specific oromandibular dystonia. Eur J Neurol 2013;20:e80. ArticlePubMed
- 38. Chidiac JJ. Oromandibular dystonia treatment following a loss of vertical dimension. Dent Update 2011;38:120–122.ArticlePubMed
- 39. Seeman MV, Clodman D, Remington G. Transient tardive dystonia: overview and case presentation. J Psychiatr Pract 2008;14:251–257.ArticlePubMed
- 40. Yoshida K. Coronoidotomy as treatment for trismus due to jaw-closing oromandibular dystonia. Mov Disord 2006;21:1028–1031.ArticlePubMed
- 41. Peñarrocha M, Sanchis JM, Rambla J, Sánchez MA. Oral rehabilitation with osseointegrated implants in a patient with oromandibular dystonia with blepharospasm (Brueghel’s syndrome): a patient history. Int J Oral Maxillofac Implants 2001;16:115–117.PubMed
- 42. Fletcher NA, Harding AE, Marsden CD. The relationship between trauma and idiopathic torsion dystonia. J Neurol Neurosurg Psychiatry 1991;54:713–717.ArticlePubMedPMC
- 43. Mokri B, Ahlskog JE, Fulgham JR, Matsumoto JY. Syndrome resembling PSP after surgical repair of ascending aorta dissection or aneurysm. Neurology 2004;62:971–973.ArticlePubMed
- 44. Tisel SM, Ahlskog JE, Duffy JR, Matsumoto JY, Josephs KA. PSP-like syndrome after aortic surgery in adults (Mokri syndrome). Neurol Clin Pract 2020;10:245–254.ArticlePubMedPMC
- 45. Lee YH, Jeong SH, Kim HJ, Hwang JM, Lee W, Kim JS. Vertical head thrusting in acquired supranuclear vertical ophthalmoplegia. J Neuroophthalmol 2017;37:386–389.ArticlePubMed
- 46. Kim EJ, Oh SY, Choi HC, Shin BS, Seo MW, Choi JB. Selective saccadic palsy after cardiac surgery. J Neuroophthalmol 2010;30:268–271.ArticlePubMed
- 47. Nandipati S, Rucker JC, Frucht SJ. Progressive supranuclear palsy-like syndrome after aortic aneurysm repair: a case series. Tremor Other Hyperkinet Mov (N Y) 2013;3:tre-03-201-4686-1.ArticlePubMedPMC
- 48. Kim EJ, Choi KD, Kim JE, Kim SJ, Kim JS, Kim JS, et al. Saccadic palsy after cardiac surgery: serial neuroimaging findings during a 6-year follow-up. J Clin Neurol 2014;10:367–370.ArticlePubMedPMC
- 49. Vaughan C, Samy H, Jain S. Selective saccadic palsy after cardiac surgery. Neurology 2008;71:1746–1747.ArticlePubMed
- 50. Eggers SD, Moster ML, Cranmer K. Selective saccadic palsy after cardiac surgery. Neurology 2008;70:318–320.ArticlePubMed
- 51. Solomon D, Ramat S, Tomsak RL, Reich SG, Shin RK, Zee DS, et al. Saccadic palsy after cardiac surgery: characteristics and pathogenesis. Ann Neurol 2008;63:355–365.ArticlePubMed
- 52. Yee RD, Purvin VA. Acquired ocular motor apraxia after aortic surgery. Trans Am Ophthalmol Soc 2007;105:152–158.PubMedPMC
- 53. Antonio-Santos A, Eggenberger ER. Asaccadia and ataxia after repair of ascending aortic aneurysm. Semin Ophthalmol 2007;22:33–34.ArticlePubMed
- 54. Kim HT, Shields S, Bhatia KP, Quinn N. Progressive supranuclear palsylike phenotype associated with bilateral hypoxic-ischemic striopallidal lesions. Mov Disord 2005;20:755–757.ArticlePubMed
- 55. Bernat JL, Lukovits TG. Syndrome resembling PSP after surgical repair of ascending aorta dissection or aneurysm. Neurology 2004;63:1141–1142.Article
- 56. Tomsak RL, Volpe BT, Stahl JS, Leigh RJ. Saccadic palsy after cardiac surgery: visual disability and rehabilitation. Ann N Y Acad Sci 2002;956:430–433.PubMed
- 57. Kupsky WJ, Drozd MA, Barlow CF. Selective injury of the globus pallidus in children with post-cardiac surgery choreic syndrome. Dev Med Child Neurol 1995;37:135–144.PubMed
- 58. Björk VO, Hultquist G. Brain damage in children after deep hypothermia for open-heart surgery. Thorax 1960;15:284–291.ArticlePMC
- 59. Greeley WJ, Ungerleider RM, Smith LR, Reves JG. The effects of deep hypothermic cardiopulmonary bypass and total circulatory arrest on cerebral blood flow in infants and children. J Thorac Cardiovasc Surg 1989;97:737–745.ArticlePubMed
- 60. Ahn JH, Song J, Choi I, Youn J, Cho JW. Risk factors and prognosis of adult-onset post-pump chorea. J Neurol Sci 2021;422:117328.ArticlePubMed
- 61. Park KW, Choi N, Ryu HS, Kim HJ, Lee CS, Chung SJ. Post‐pump chorea and progressive supranuclear palsy‐like syndrome following major cardiac surgery. Mov Disord Clin Pract 2020;7:78–82.ArticlePubMedPDF
- 62. Bisciglia M, London F, Hulin J, Peeters A, Ivanoiu A, Jeanjean A. Choreoathetotic syndrome following cardiac surgery. J Clin Anesth 2017;36:59–61.ArticlePubMed
- 63. Hamzi MA, Hassani K, El Kabbaj D. Late-onset choreoathetotic syndrome following heart surgery in adults with end-stage renal disease. Saudi J Kidney Dis Transpl 2018;29:202–206.ArticlePubMed
- 64. Passarin MG, Romito S, Avesani M, Alessandrini F, Petrilli G, Santini F, et al. Late-onset choreoathetotic syndrome following heart surgery. Neurol Sci 2010;31:95–97.ArticlePubMedPDF
- 65. Khan A, Hussain N, Gosalakkal J. Post-pump chorea: choreoathetosis after cardiac surgery with hypothermia and extracorporeal circulation. J Pediatric Neurol 2012;10:57–61.
- 66. Medlock MD, Cruse RS, Winek SJ, Geiss DM, Horndasch RL, Schultz DL, et al. A 10-year experience with postpump chorea. Ann Neurol 1993;34:820–826.ArticlePubMed
- 67. Aoyagi K, Higuchi Y, Okahara Y, Yakufujiang M, Matsuda T, Yamanaka Y, et al. Effects of bilateral pallidal deep brain stimulation on chorea after pulmonary thromboendarterectomy with deep hypothermia and circulatory arrest: a case report. Acta Neurochir (Wien) 2018;160:393–395.ArticlePubMedPDF
- 68. Youn J, Todisco M, Zappia M, Pacchetti C, Fasano A. Parkinsonism and cerebrospinal fluid disorders. J Neurol Sci 2022;433:120019.ArticlePubMed
- 69. Prashantha DK, Netravathi M, Ravishankar S, Panda S, Pal PK. Reversible parkinsonism following ventriculoperitoneal shunt in a patient with obstructive hydrocephalus secondary to intraventricular neurocysticercosis. Clin Neurol Neurosurg 2008;110:718–721.ArticlePubMed
- 70. Zeidler M, Dorman PJ, Ferguson IT, Bateman DE. Parkinsonism associated with obstructive hydrocephalus due to idiopathic aqueductal stenosis. J Neurol Neurosurg Psychiatry 1998;64:657–659.ArticlePubMedPMC
- 71. Curran T, Lang AE. Parkinsonian syndromes associated with hydrocephalus: case reports, a review of the literature, and pathophysiological hypotheses. Mov Disord 1994;9:508–520.ArticlePubMed
- 72. Gatto M, Micheli F, Pardal MF. Blepharoclonus and parkinsonism associated with aqueductal stenosis. Mov Disord 1990;5:310–313.ArticlePubMed
- 73. Shahar E, Lambert R, Hwang PA, Hoffman HJ. Obstructive hydrocephalus-induced parkinsonism. I: decreased basal ganglia regional blood flow. Pediatr Neurol 1988;4:117–119.ArticlePubMed
- 74. Berger L, Gauthier S, Leblanc R. Akinetic mutism and parkinsonism associated with obstructive hydrocephalus. Can J Neurol Sci 1985;12:255–258.ArticlePubMed
- 75. Brazin ME, Epstein LG. Reversible parkinsonism from shunt failure. Pediatr Neurol 1985;1:306–307.ArticlePubMed
- 76. Kim MJ, Chung SJ, Sung YH, Lee MC, Im JH. Levodopa-responsive parkinsonism associated with hydrocephalus. Mov Disord 2006;21:1279–1281.ArticlePubMed
- 77. Bhattacharjee S, Kumar H, Tiwari M, Mallick S. Hemiparkinsonism due to coiled posterior cerebral artery aneurysm. Can J Neurol Sci 2013;40:101–103.ArticlePubMed
- 78. Norris SA, Derdeyn CP, Perlmutter JS. Levodopa-responsive hemiparkinsonism secondary to cystic expansion from a coiled cerebral aneurysm. J Neuroimaging 2015;25:316–318.ArticlePubMedPDF
- 79. Pande S, Ang K, Myat MW, Neo S, Subramaniam S. Spinal segmental myoclonus following spinal surgery. Br J Neurosurg 2020;Jun. 12. [Epub]. Available from: https://doi.org/10.1080/02688697.2020.1777262. Article
- 80. Sardana V, Sharma SK. Delayed propriospinal myoclonus following dorsal spinal cord surgery. Ann Indian Acad Neurol 2019;22:491–493.ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

- Biofeedback Endurance Training for Gait Rehabilitation in Parkinson’s Disease: a Non-Randomized Controlled Study
Olga V. Guseva, Natalia G. Zhukova
Bulletin of Rehabilitation Medicine.2024; 22(6): 21. CrossRef
Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite