Parkinson’s Disease in Sub-Saharan Africa: A Review of Epidemiology, Genetics and Access to Care
Article information
Abstract
A low prevalence of Parkinson’s disease (PD) has been reported in the Sub-Saharan Africa (SSA) region. The genetic causes and clinical features of PD in this region have been poorly described. Very few reports have examined the availability and access to evidence-based quality care for people living with PD in this region. We reviewed all publications focusing on idiopathic PD from SSA published up to May 2016 and observed a prevalence of PD ranging from 7/100,000 in Ethiopia to 67/100,000 in Nigeria. The most recent community-based study reported a mean age at onset of 69.4 years. The infrequent occurrence of mutations in established PD genes was also observed in the region. Treatments were non-existent or at best irregular. Additionally, there is a lack of well-trained medical personnel and multidisciplinary teams in most countries in this region. Drugs for treating PD are either not available or unaffordable. Large-scale genetic and epidemiological studies are therefore needed in SSA to provide further insights into the roles of genetics and other etiological factors in the pathogenesis of PD. The quality of care also requires urgent improvement to meet the basic level of care required by PD patients.
INTRODUCTION
Sub-Saharan Africa (SSA) is composed of African countries south of the Sahara and consists of 47 politically distinct states. SSA is one of the fastest growing regions of the world, with significant economic growth in the past 10–15 years, which has reflected in a greater availability of and access to health facilities. However, most countries in the region are still below the World Health Organization (WHO) standard of one doctor per 100,000 population [1]. The population of those with Parkinson’s disease (PD) in the SSA region is known to be relatively small. The low prevalence of PD in SSA may stem from several issues, including the relatively youthful population in SSA, with less than 5% of the total population above 65 years; the fact that most cases of PD are not recognized partly because of the assumption that neurological diseases are part of normal aging; and the scarcity of epidemiological studies in SSA [2].
Key aspects of PD, such as the incidence and prevalence, genetic causes and clinical presentation, are poorly characterized in SSA [3]. Few reports have examined the availability of and access to evidencebased quality care for people living with PD in SSA countries.
This review aims to accomplish the following:
1) Provide a descriptive summary of all epidemiologic and genetic studies from SSA published up to May 2016.
2) Compare the genetic and epidemiologic results from SSA to those from other populations in Africa outside SSA.
3) Review the level of care available and accessible to PD patients in SSA.
MATERIALS & METHODS
Inclusion criteria
This review included all population-, outpatient clinic- and in-patient-based studies investigating epidemiologic, genetic or care-related aspects of PD in SSA up to May 2016. Only studies focusing on idiopathic PD using either the UK Parkinson Disease Society (UKPDS) brain bank [4] or the WHO or Movement Disorders Society (MDS) diagnostic criteria [5] were included.
Exclusion criteria
Only studies published in English were included. Articles on neurological inpatients or outpatients that did not focus on PD frequencies were excluded. We excluded review articles, with the exception of their references.
Search methods
Electronic searches
Electronic databases, such as MEDLINE and African journals online, were used as the initial search resources. To include all relevant publications within the scope of this review, the search was extended to include the following:
1) Reference lists of all review articles and primary studies available.
2) References found in abstract books of the European Federation of Neurological Societies (EFNS), American Academy of Neurology (AAN), MDS, and Pan-African Association of Neurological Societies (PAANS).
3) References lists of relevant textbooks and citations of PD studies in Africa.
If articles investigating the treatment available for PD patients in SSA were not found in the initial search resources, the search was extended to include available WHO and World Bank data containing statistics on health care personnel and facilities in different regions of the world. Websites of the MDS Africa task force, patient support groups (Parkinson’s Disease Association) and professional organizations (e.g., speech and language therapy and occupational therapy) in Africa were also searched for available relevant services. Reports of PD-related services were also evaluated in this review. The flow diagram of the search process is shown in Figure 1.
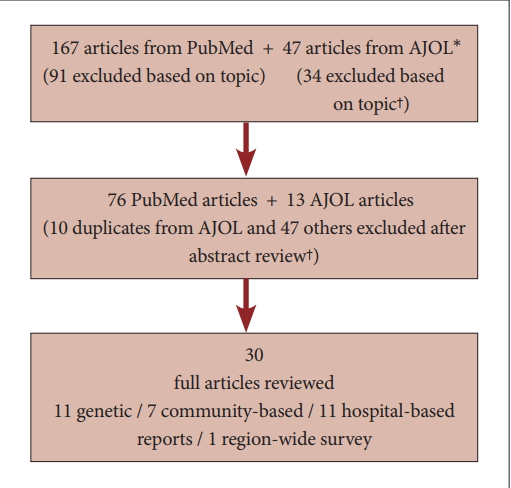
Flow diagram of search process. *African journal online (AJOL) is a collection over 350 journals with focus on research from African academics. † articles excluded based on topic if the topic did not suggest the paper is focusing on epidemiology, genetics or care issues in Sub-Saharan Africa. Those excluded based on abstract are those whose topic may suggest a focus that is relevant to the review but the abstract indicates it does not meet the inclusion criteria.
Data collection and analysis
Study selection
The titles and abstracts obtained from the electronic database search were reviewed, and irrelevant studies were excluded. Full copies of the relevant studies were obtained and reviewed in detail.
Data extraction
Relevant information from the studies was obtained using a data extraction sheet. The information sought included year of study, authors, country, setting of study, duration of study, year of publication, type of study, number of subjects, study design, characteristics of participants [age, gender, age at onset, duration of disease, Hoehn and Yahr rating, UPDRS (unified Parkinson’s disease rating scale) score, family history of PD], description of the study, and diagnostic criteria used. For prevalence studies, the crude prevalence and age-adjusted prevalence were recorded where available and reported as the number of patients per 100,000 persons. In the case of genetic studies, information regarding the genes studied, genetic mutations found, and the method for genetic analysis used was also extracted. Studies in other populations with similar characteristics were reviewed for prevalence statistics for comparison with those obtained in SSA.
Data analysis
Data analysis was performed using Microsoft Excel, and the results are presented in the text, figures and tables.
RESULTS
We identified 11 genetic studies, 7 communitybased (door-to-door) prevalence studies and no incidence studies in SSA populations. The genetic studies were all undertaken within the past decade. Regarding the distribution of genetic studies, 7 were from South Africa and 1 each was from Nigeria, Zambia and Ghana. One study [6] included samples from the Human Diversity series extracted from different countries in SSA, including Nigeria, the Central African Republic, the Democratic Republic of the Congo, Kenya, Namibia, Senegal and South Africa. The first genetic study was conducted by Okubadejo et al. [7], in which they screened 51 Nigerians with sporadic PD and an equal number of controls for mutations in leucine-rich repeat kinase 2 (LRRK2), parkin (PRKN) and ataxin 3 (ATXN3). They concluded that pathogenic mutations in these genes were not common in Nigerians. Studies from South Africa examined mutations involving eukaryotic translation initiation factor 4GI (EIF4GI), vacuolar protein sorting 35 (VPS35), Parkin, microtubule-associated protein tau (MAPT), synuclein alpha interaction protein (SNCAIP), PTEN-induced putative kinase 1 (PINK1), LRRK2, SNCA, PINK1, DJ-1, ubiquitin carboxyl-terminal esterase L1 (UCH-L1), ATP13A2, LPA, TNFSF9, CAV2, CAV1, and GCH1 [8-12]. These studies concluded that mutations in these genes are not a common cause of PD in the South African population.
The Ghanaian study analyzed mutations in the LRRK2 gene, while that from Zambia screened all the SNCA, LRRK2, PINK1, DJ-1, and Parkin exons for any pathogenic mutations. Interestingly, these studies did not detect any pathogenic mutations among the indigenous black PD patients compared to other populations in which mutations in these genes have been associated with either sporadic or familial PD. The findings from these genetic studies are summarized in Table 1 and 2.
The fact that these mutations were not observed in any of the genetic screens in SSA suggests that PD in SSA may have a unique genetic heritage. The genetic architecture of the indigenous people of SSA appears to be different from that of other populations outside Africa and in parts of Africa outside SSA. Despite the progress made in the past decade in PD genetics in SSA, it remains largely unexplored despite offering potentially one of the greatest diversity of polymorphisms that can be studied in any region of the world.
Prevalence of PD in SSA
The first epidemiological studies on PD in SSA were undertaken in the 1980s in Nigeria, Ethiopia and Togo, and the most recent studies were community-based prevalence studies from SSA, as summarized in Table 3. The prevalence of PD in these studies ranged from 7/100,000 in Ethiopia to 67/100,000 in Nigeria. Data from the World Bank regarding the estimated proportion of the country older than 65 years at the times these studies were conducted are similar, suggesting a similarity in population structure in the different SSA countries studied [13]. The first large-scale community-based prevalence study of PD was undertaken by Dotchin et al. [14] This study investigated a population of 161,071 community dwellers using a two-stage procedure that involved 6 initial screening sessions followed by an expanded history and neurological examination of positive responders to confirm the diagnosis of PD. They utilized the UKPDS brain bank criteria for the diagnosis of PD [4,15]. The age-adjusted prevalence was 40/100,000, which is lower than figures from Sweden and the United States, which were 76/100,000 and 1036-2168/100,000, respectively [16,17].
Hospital-based studies
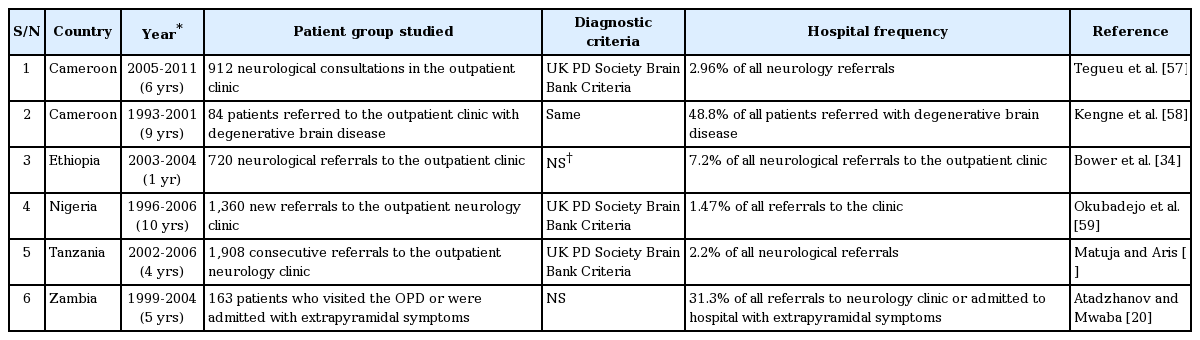
Several clinic- and hospital-based studies have estimated the frequency of PD in SSA among medical admissions, neurological admissions, or neurology clinic attendants. Unlike community-based studies, most hospital-based series are recent surveys undertaken within the last 10 years. The hospital frequency depends on the setting in which it was estimated, as in-patient-based admission estimates are lower than ones from outpatient-based studies. Only two admission-based studies were identified. Talabi [18] reported that PD accounted for 0.5% of all neurological admissions in a Nigerian hospital, while Lombard and Gelfand [19] reported that PD was responsible for 0.02% of admissions among black populations in a Zimbabwean hospital between 1973 and 1976. Outpatient-based studies reviewing hospital records of consecutive referrals to neurology clinics provided estimates of PD frequency among neurology referrals or patients with Parkinsonism. The outpatient-based series and the estimated hospital frequency of PD studies and their references are summarized in Table 4.
Clinical characteristics of the different cohorts of patients studied in the sub-Saharan Africa studies
Dotchin et al. [14] reported a mean age at disease onset of 69.4 years in the most recent communitybased population prevalence survey. The age of the patients at the time of these studies ranged from as young as 17 years, reported by Atadzhanov and Mwaba [20], to as old as 94 years, reported by Dotchin et al. [14] These studies, in keeping with reports in other populations outside SSA, observed a male preponderance, with a male-to-female ratio ranging from 1.2:1 to 4:1.
Evaluation of available care for PD patients in SSA
Evaluating the care of patients with PD in SSA is very challenging, as there are no systematic studies evaluating the care available or accessible to PD patients in this area. This review compares the availability of personnel, drugs and facilities in different settings to that recommended by standard guidelines such as the National Institute for Health and Care Excellence (NICE) [21] or the Canadian guidelines for PD [22].
Availability of standard management for PD patients in SSA
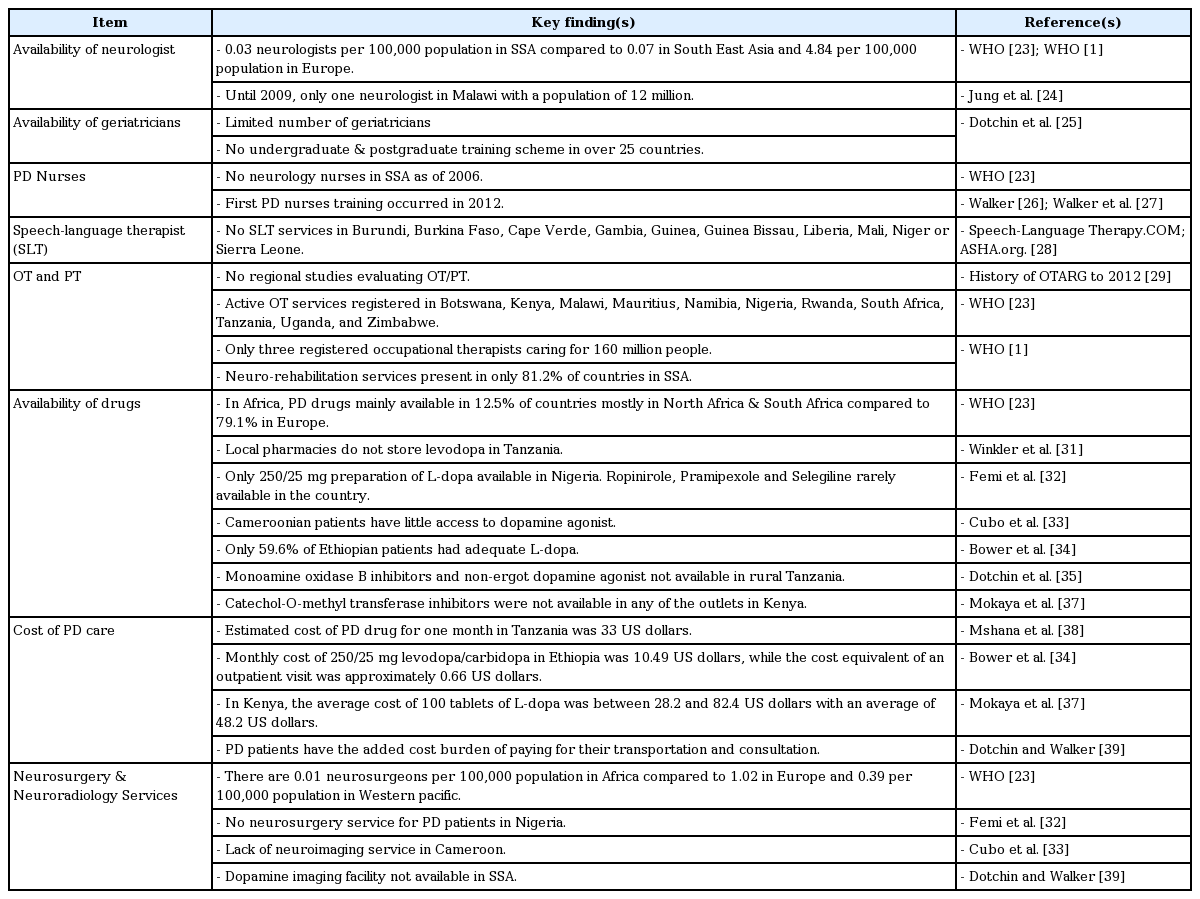
Neurologists
The lack of neurologists and movement disorder specialists has made it difficult to diagnose and treat PD patients properly in SSA. Documented estimates of country medical and neurological resources reported in the WHO atlas of country resources for neurological disorders [23] and WHO reports [1] indicate that there are 0.03 neurologists per 100,000 population compared to 0.07 in South East Asia and 4.84 per 100,000 population in Europe. Looking at the mean average of neurologists for all SSA countries does not portray the real deficiencies in the different countries. A typical example is Malawi, where it was reported that until recently, there was only one part-time neurologist for a population of approximately 12 million people [24].
Moreover, the number of geriatricians was small in SSA, with over 30 countries having no undergraduate or postgraduate geriatric training scheme [25].
Parkinson’s disease nurses
PD is a new field of specialization in the region, as the first ever PD nurse training program in the region was led by Louise Ebenezer, a PD nurse specialist from the United Kingdom, in Tanzania in December 2012 with participating nurses from Rwanda, Uganda, Ethiopia, Kenya, Nigeria and Tanzania [26,27]. Subsequent courses have been held in Anglophone West Africa (Ghana 2013), Southern Africa (South Africa 2014) and Eastern Africa (Ethiopia in January 2016). WHO data show that there were no neurology nurses as of 2006 in SSA per 100,000 population compared to 0.005 in Southeast Asia, 0.13 in the Eastern Mediterranean, 0.14 in the Americas, 0.32 in the Western Pacific and 2.43 in Europe per 100,000 population at the same time [23].
Speech-language therapist
The presence of speech-language therapist (SLT) services in each country from SSA was assessed to evaluate their availability. A search of the SSA regional SLT website suggests that no SLT services have been registered in the following countries: Burundi, Burkina Faso, Cape Verde, Gambia, Guinea, Guinea Bissau, Liberia, Mali, Niger and Sierra Leone.
SLT services are available in Kenya, Rwanda, Tanzania and Uganda in East Africa and in Cote d’Ivoire, Ghana, Nigeria, Senegal and Togo in West Africa; additionally, training is available at Kampala, Uganda, and at several universities in South Africa, including the university of Pretoria, Witwatersrand, Limpopo, Cape Town, and Stellenbosch [28].
Occupational therapy and physiotherapy
No regional studies have evaluated the availability or benefit of occupational therapy (OT)/physiotherapy (PT) services among Africans with PD. Active OT services are, however, currently registered in Botswana, Kenya, Malawi, Mauritius, Namibia, Nigeria, Rwanda, South Africa, Tanzania, Uganda, and Zimbabwe [29]. For example, Nigeria had only 3 registered occupational therapists out of 160 million people [23]. As of 2006, WHO data suggest that neuro-rehabilitation services are generally present in 81.2% of countries in Africa [1]. Dotchin et al. [30] reported that none of the PD patients reviewed in their series had visited a physiotherapist, while only one patient had seen an occupational therapist.
Availability of drugs
Most patients in SSA with PD do not have access to PD medication due to unavailability or, where available, financial constraints. PD drugs are available in only 12.5% of Africa, mostly in North Africa and South Africa, compared to 79.1% in Europe [23]. Indeed, studies from SSA referring to the availability of PD drugs indicate that procurement of Parkinsonism drugs remains a difficult task. Winkler et al. [31] stated that local pharmacies did not store L-dopa. Furthermore, the nearest pharmacy with a supply of L-dopa available was approximately 300 km away, thus introducing the additional cost of transportation, which most of the patients could not afford. In northern Nigeria, only 250/25 mg of L-dopa was available to PD patients [32]. Patients in their study who were able to afford ropinirole, pramipexole and selegiline had these medications brought in from either the Middle East or Asia. Cubo et al. [33] compared the clinical profile of PD patients in Spanish and Cameroonian cohorts. They observed that the Cameroonian patients were intermittently treated, received L-dopa at lower doses, had less access to DAs, and used anticholinergics more frequently. The use of selegiline, rasagiline and DAs was noted mostly in the Spanish cohort. Bower et al. [34] evaluated the adequacy of treatment of PD patients seen in a movement disorders clinic in Ethiopia and reported that only 59.6% of their patients received adequate L-dopa over the study period.
Dotchin et al. [35] investigated levodopa plus carbidopa use in a three-year follow-up study in Hai in rural Tanzania. They reported that MAO-B inhibitors and non-ergot DA were unavailable to PD patients. Bromocriptine, an ergot DA, was available as it was on the essential medicines list for the treatment of infertility. Ogunniyi [36], in his review titled, “Treatment of Parkinsonism syndromes in developing countries,” observed that anticholinergics are among the most commonly used drugs and that the use of DAs is limited due to their high cost. Overall, the literature suggests that even when patients are able to be diagnosed with PD, treatment is irregular or non-existent, monitoring is limited, people resort to local traditional healers for treatment, and multidisciplinary staff are rarely available [30]. Mokaya et al. [37] evaluated 48 drug outlets in Kenya and reported that only 24 outlets, one of which was a public pharmacy, had L-dopa. Ergot-derived DAs and anticholinergics were sold in 37 and 35 outlets, respectively. Catechol-O-methyl transferase inhibitors were not available in any of the outlets.
The cost burden of PD management in SSA
Although the estimated cost burden of the treatment of PD varied by country, it was generally accepted that the cost burden was comparably high, making it difficult for most PD patients in SSA to maintain treatment. In Tanzania, Mshana et al. [38] estimated that a one-month supply of PD drugs cost approximately 40,000 Tanzanian shillings, which is equivalent to approximately 33 US dollars (USD). In Ethiopia, Bower et al. [34] reported that a monthly dose of 25 mg/250 mg levodopa/carbidopa cost an estimated 90 Ethiopian birr, which is equivalent to 10.49 USD, and that the cost of an outpatient visit was approximately 0.66 USD. In Kenya, 100 tablets of L-dopa cost between 28.2 and 82.4 USD, with a mean cost of 48.2 USD [37].
In many SSA settings, patients also pay for their transportation and for the consultation, thereby increasing the cost of PD care [39]. Cilia et al. [40] observed that when they compared Ghanaian and Italian PD patients, levodopa was introduced to patients in Ghana later than to the Italian patients.
Neurosurgery and neuroradiology services
The estimated median number of neurosurgeons per 100,000 population is 0.01 in Africa compared to 1.02 in Europe and 0.39 per 100,000 population in the Western Pacific [23]. Femi et al. [32] reported that there were no neurosurgical services available for PD patients in a hospital-based clinical series in Nigeria. Cubo et al. [33] reported that a lack of imaging facilities impaired their ability to differentiate secondary Parkinsonism from idiopathic PD among the patients they were evaluating in Cameroon. Dechambenoit et al. [41], in his paper “Action Africa,” described a lack of equipment and outdated operating theaters, with an absence of instruments for microsurgery and microscopes. He further stated that where neurosurgery institutes were available, they were inaccessible to 90% of the population due to a lack of health insurance systems. He also expressed the view that the prevailing economic conditions in SSA underlie the difficulties in equipping neurology and neurosurgical services, as well as the training of young doctors. His views regarding the lack of neuroimaging services were supported by Dotchin and Walker [39], who reported that dopamine transporter imaging, which is useful in differentiating PD from other tremor disorders, is unavailable in all settings in SSA. A summary of available standard management of PD patients in SSA is presented in Table 5.
DISCUSSION
Genetics of PD in SSA
The findings of this review support the view that there is a possible unique genetic architecture of PD in the region because of the infrequent occurrence of mutations in established PD genes in SSA. This interpretation must, however, be viewed in the context that most of the studies in SSA had small sample sizes, with the largest being 205 subjects [12], which makes the generalization of their findings difficult. The likelihood of discovering mutations in PARK genes might increase if considerably larger PD cohorts from SSA were to be analyzed. The identification of mutations in established PD genes was documented only for Parkin among South African and Zambian cohorts from the 11 studies reviewed [9,42,43]. Both homozygous exon deletions [9,43] and point mutations [9,42,43] were observed in these studies. No pathogenic mutations were documented in studies screening for universally recognized PD genes such as α-synuclein, LRRK2, PINK1 and DJ-1 or genes of uncertain significance such as UCHL-1 in sub-Saharan PD cohorts. The mean age at onset in most of the patients studied was above 50 years, while mutations in PD genes are more commonly found in early-onset PD, especially mutations in autosomal recessively inherited genes such as Parkin, PINK1 and DJ-1.
Epidemiology
This review has described different rates of occurrence in both hospital- and community-based studies. Balogou et al. [44] reported a prevalence rate of 20/100,000 in their study, but Winkler et al. [31] found no cases of PD among 1,569 community dwellers screened in rural Tanzania. The findings from other populations are significantly higher than those reported in SSA. Seo et al. [45] in Korea reported a prevalence of 374/100,000 people > 18 years, while a prevalence of 146/100,000 was reported in Australia [46]. These findings are very close to those of different European studies, such as the prevalence of 144/100,000 in Wales [47], 128/100,000 in England [48] and 166/100,000 in Finland [49]. Mayeux et al. [50] published a slightly lower prevalence of 107/100,000 in the United States of America. The lower prevalence of PD in SSA could be due to different study methodologies and diagnostic criteria; the lower proportion of those older than 65 years in the SSA population; reduced access to health care; different health-seeking behavior in the region; disease-related disability and cultural differences in the studied populations [3]; or the underdiagnosis of PD due to low social recognition, as typical symptoms of PD might be mistaken for symptoms of normal aging [51]. Blanckenberg et al. [3] suggested that the decreased survival of PD patients due to a lack of drugs, unavailability of proper health care and other socio-economic challenges might also result in a lower prevalence. The possibility of as yet undefined genetic and environmental factors has also been suggested as a possible reason for the lower prevalence of PD in SSA [2].
It is difficult to draw much inference from, or to compare, hospital-based series in SSA because of the marked difference in methodology applied regarding diagnostic criteria, population characteristics, and the estimated proportion reported, as some studies did not discriminate between idiopathic PD and other causes of secondary Parkinsonism. Door-to-door surveys offer the best estimate of PD prevalence because they can detect as yet undiagnosed cases in the community among people with different health-seeking behaviors. However, a major challenge to this type of study is the high cost, which is inherently a major factor affecting research in SSA.
Care availability and accessibility
Even when PD patients are able to be diagnosed, treatment is often non-existent or irregular; monitoring is limited; multidisciplinary teams are rarely available; and patients often turn to local traditional healers for treatment [13]. The other challenge high-lighted is the paucity of neurologists and other health care personnel caring for PD patients. The situation is the same for neurosurgeons, neuroradiologists, geriatricians, PD nurses and other health care personnel. The treatment of patients after diagnosis is hampered by a lack of drugs, as these drugs are unavailable or unaffordable to the patients. Some of the available drugs are no longer used as first-line drug treatment in Europe and in other countries with a higher GDP and better-developed neurological services. For instance, anticholinergics and ergot DAs were used more than non-ergot DAs and MAO-B inhibitors in SSA. The impact of the high cost of PD treatment on access to PD drugs cannot be over-emphasized in a region where almost 50% of the population lives on less than 1.25 USD per day [52]. The location of the few health facilities being far away from patients’ residences again increases the financial burden on the patient, as they have to pay for the cost of transportation and consultation out of pocket in a region where only a small minority have health insurance.
CONCLUSION
This review has highlighted the challenges involved in conducting genetic and epidemiologic studies in SSA. It has also been shown that the currently available care for PD patients is grossly inadequate. Large-scale, state-of-the-art genetic and epidemiological studies will help to provide further insight into the role of genetic factors and other etiological factors such as toxin exposure in PD in SSA. Collaborative studies using the same methodology and staffed by personnel with comparable training will help establish a data set that could then be used to compare the PD prevalence rates across different populations. The establishment of minimal consensus management guidelines may help to improve the consistency and quality of care. International assistance is needed regarding improved public awareness of neurological diseases, training of health care workers, provision of affordable drugs and facilities for PD treatment and the monitoring of PD patients in the region.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.