The Non-Motor Symptom Profile of Progressive Supranuclear Palsy
Article information
Abstract
Objective
Non-motor symptoms (NMSs) significantly contribute to increased morbidity and poor quality of life in patients with parkinsonian disorders. This study aims to explore the profile of NMSs in patients with progressive supranuclear palsy (PSP) using the validated Non-Motor Symptom Scale (NMSS).
Methods
Seventy-six patients with PSP were evaluated in this study. Motor symptoms and NMSs were evaluated using the PSP Rating Scale (PSPRS), Unified Parkinson’s Disease Rating Scale-III, Montreal Cognitive Assessment, Hamilton Depression (HAM-D) and Anxiety Rating Scales, Parkinson’s Disease Sleep Scale (PDSS) and NMSS. NMS severity and prevalence were also compared between patients with PSP-Richardson syndrome (PSP-RS) and those with PSP-parkinsonism.
Results
All subjects in this cohort reported at least 2 NMSs. The most prevalent NMSs in patients with PSP were in the domains of sleep/fatigue, mood/cognition, and sexual function. The least prevalent NMSs were in the domains of cardiovascular including falls, and perceptual problems/hallucinations. Significant correlations were observed between the NMSS scores and HAM-D, PDSS, PSPRS scores and PSPRS sub-scores. The severity of NMSs was unrelated to the duration of illness. Patients with PSP-RS reported a higher severity of drooling, altered smell/taste, depression and altered interest in sex and a higher prevalence of sexual dysfunction.
Conclusion
NMSs are commonly observed in patients with PSP, and the domains of sleep, mood and sexual function are most commonly affected. These symptoms contribute significantly to disease morbidity, and clinicians should pay adequate attention to identifying and addressing these symptoms.
Over the past decade, the concepts of the symptomatology of parkinsonian disorders have undergone a significant change owing to the recognition of non-motor symptoms (NMSs) in these disorders. A vast majority of studies exploring NMSs in parkinsonian disorders have focused on the assessment of NMSs in Parkinson’s disease (PD), and these have been reported to involve several domains, such as sleep disturbances, cognition, psychiatric and behavioral problems, gastrointestinal problems, urinary disturbances, and sexual dysfunction [1]. These symptoms significantly impair the quality of life and increase morbidity and disability in PD [2]. It has been suggested that in PD, in addition to clinical motor symptom staging, NMSs should also be classified [3]. Although extensively studied in PD [4-6], relatively few studies have explored the extent and impact of NMSs in other parkinsonian disorders, such as multiple system atrophy and progressive supranuclear palsy (PSP) [7-11].
PSP is an atypical parkinsonian disorder typically characterized by progressive postural instability with falls, supranuclear vertical gaze palsy, pseudobulbar palsy, levodopa-unresponsive parkinsonism, and frontal cognitive disturbances [12]. Patients with PSP are known to develop neuropsychiatric symptoms such as apathy and depression [13]. In addition, a wide range of other NMSs have also been reported in patients with PSP. These predominantly include the domains of sleep and fatigue, mood and cognition, and urinary dysfunction [7-11]. Although several studies have attempted to explore the prevalence and frequency of NMSs in patients with PSP, there is a significant variability in the patterns of prevalence of NMSs observed across studies. For instance, the Parkinson and non-motor symptoms (PRIAMO) study utilized a structured questionnaire to evaluate NMSs in parkinsonian disorders. This cohort included 30 patients with PSP, in addition to patients with multiple system atrophy (n = 34), corticobasal degeneration (n = 11), dementia with Lewy bodies (n = 14) and vascular parkinsonism (n = 83). They reported gastrointestinal problems and fatigue as the most prevalent NMSs in PSP [14]. A recent study by Radicati et al. [7] utilized the NMSS on 50 patients with PSP and 100 patients with PD and reported urinary dysfunction and sleep/fatigue as the most prevalent NMSs in PSP. The variability in results could be attributed to differences in the size of patient groups and the methods of estimation of NMSs.
This study aims to explore the profile of NMSs in patients with PSP using the Non-Motor Symptom Scale (NMSS) [15], to compare NMSs between patients with PSP-Richardson syndrome (PSPRS) and those with PSP-parkinsonism (PSP-P), and to identify the clinical correlates of NMSs in PSP. Additionally, we attempted to ascertain the impact of NMSs on caregiver burden.
MATERIALS & METHODS
Subject recruitment
This was a prospective study conducted over 10 months at the National Institute of Mental Health and Neurosciences (NIMHANS), India. Consecutive patients diagnosed with probable or possible PSP, as per the Movement Disorder Society criteria for PSP [16], by a qualified movement disorder specialist were recruited from the general neurology outpatient and movement disorder clinics at NIMHANS. This study was approved by the institute’s ethics committee [NO. NIMH/DO/IEC (BS&NS DIV)/2017-18], and informed consent was obtained from all subjects.
Assessments
Demographic details such as age, age at onset and the duration of illness were recorded. The rating of symptom severity and the staging of PSP were performed using the PSP Rating Scale (PSPRS) [17]. The Unified Parkinson’s Disease Rating Scale III (UPDRS-III) was also applied in the off state (at least 12 hours after the last dose of levodopa, 48 hours after the last dose of a dopamine agonist) [18]. The Hamilton Anxiety Rating Scale (HAM-A) and Hamilton Depression Rating Scale (HAM-D) were administered to estimate the presence and severity of anxiety and depression, respectively. The severity of sleep disturbances was assessed using the Parkinson’s Disease Sleep Scale (PDSS), and the Montreal Cognitive Assessment Scale (MoCA) was applied to assess cognitive function. The severity and frequency of NMSs was assessed by using the validated NMSS [15]. This is a 30-item scale covering 9 domains of NMSs: cardiovascular (CVS), including falls; sleep/fatigue; mood/cognition; perceptual problems/hallucinations; attention/memory; gastrointestinal tract; urinary; sexual function; and miscellaneous. The severity (0–3), frequency (1–4) and final score (severity × frequency) of each item are evaluated separately, and the total score for a domain is obtained by the sum of individual item scores. The total NMSS score ranges from 0 to 320, and higher scores are indicative of a higher severity and frequency of NMSs. The NMS burden was estimated using burden grading cut-off scores, i.e., 0: none, 1–20: mild, 21–40: moderate, 41–70: severe, and ≥ 71: very severe [2]. Finally, caregiver burden was estimated using the Zarit Burden interview [19].
Statistical analysis
Descriptive statistical analysis was performed for the demographic and clinical features of the entire cohort of patients with PSP. Data were tested for normality, following which the PSP-RS and PSP-P subgroups were compared. The third subgroup comprised other subtypes and was excluded from comparison owing to the small sample size. Partial correlations with age as a covariate were performed between scores of the NMSS domains, clinical parameters, PSPRS scores, PSPRS subscores and scores of other scales. A correlation coefficient of r > 0.5 was considered significant, and linear regressions were performed on these variables to ascertain the strength of association. Statistical significance was set at p < 0.05.
RESULTS
Demographic and clinical features
A total of 76 patients with PSP, 53 men and 23 women, were recruited for this study (Table 1). The mean age of the PSP cohort was 62.04 ± 7.10 years, with a mean disease duration of 2.68 ± 2.10 years. The cohort comprised patients with PSP-RS (n = 53, 69.73%), PSP-P (n = 16, 21.05%), PSP-with cognitive or behavioral symptoms (n = 4, 5.26%), PSP with progressive gait freezing (n = 2, 2.63%) and PSP with corticobasal syndrome (n = 1, 1.31%).
The UPDRS-III off state score was 33.08 ± 14.00, the PSPRS score was 38.97 ± 14.00, and the PSP stage was 3.13 ± 0.94. The mean MoCA score was 19.00 ± 6.60, the HAM-A score was 5.41 ± 6.30, the HAM-D score was 7.47 ± 5.90, and the PDSS score was 128.49 ± 18.10. The caregiver burden score was 21.88 ± 12.5. A comparison of the PSP-RS and PSP-P subgroups revealed a longer duration of illness in the PSP-P subgroup. No other significant differences were observed in the demographic and basic clinical scores of these two groups.
Non-motor symptom profile
Complete PSP cohort
The total NMSS score was 47.08 ± 32.40, and the NMSS burden was mild in 19.7% (n = 15), moderate in 27.63% (n = 21), severe in 35.5% (n = 27) and very severe in 17.1% (n = 13) of patients with PSP (Table 2). The mean number of NMS domains affected was 4.81 ± 1.65 (2–9 domains), and the mean number of NMSs reported was 9.61 ± 4.84 (2–24 symptoms).
The most prevalent domains of NMSs were sleep/fatigue (82.90%), mood/cognition (72.40%), and sexual function (70.40%) (Figure 1). Based on mean NMSS domain scores, the most affected domains of NMSs were mood/cognition, sleep/fatigue, and urinary (Table 2). Of the 76 patients with PSP, only 54 patients were sexually active. In the remaining 22 patients, the lack of sexual activity was unrelated to sexual dysfunction. Perceptual problems/hallucinations were the least prevalent NMSs (19.70%), followed by NMSs in the CVS system, including falls, domain (27.60%). The most prevalent item was “Does fatigue or lack of energy limit the patient’s daytime activities?”, which was reported by 65.80% of patients with PSP (Table 2).
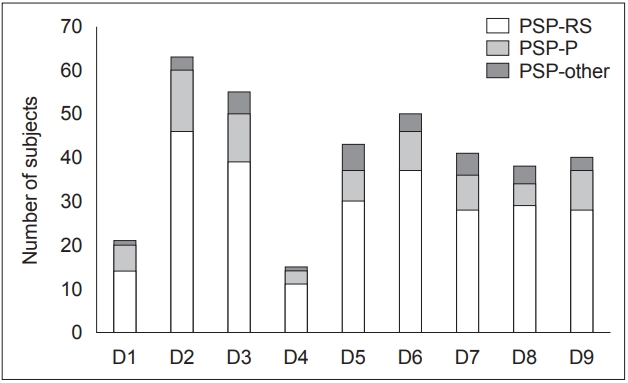
Plot demonstrating the number of subjects with a positive history of non-motor symptoms in PSP-RS, PSP-P, and PSP-other. The y axis is representative of the number of subjects, and the x axis represents the NMSS domains. D1: cardiovascular including falls, D2: sleep/fatigue, D3: mood/cognition, D4: perceptual problems/hallucinations, D5: attention/memory, D6: gastrointestinal tract, D7: urinary, D8: sexual function, D9: miscellaneous. NMSS: Non-Motor Symptom Scale, PSP: progressive supranuclear palsy, PSP-P: PSP-parkinsonism, PSP-RS: PSP-Richardson syndrome.
PSP-RS vs. PSP-P
There was no difference in the total NMSS scores between the two subgroups. In the PSP-RS subgroup, the most prevalent domains of NMSs were sleep/fatigue (86.79%), sexual function (80.56%), and mood/cognition (73.58%). Perceptual problems/hallucinations were the least prevalent NMSs (20.75%), followed by NMSs in the CVS system, including falls, domain (26.42%). In the PSP-P subgroup, the most prevalent domains of NMSs were sleep/fatigue (87.50%), mood/cognition (68.75%), and gastrointestinal tract (56.25%). Similar to the PSP-RS subgroup, perceptual problems/hallucinations were the least prevalent NMSs (18.75%), followed by NMSs in the CVS system, including falls, domain (37.50%). In both subgroups, the most prevalent item was “Does fatigue or lack of energy limit the patient’s daytime activities?”, which was reported by 69.81% of patients with PSPRS and 68.75% of patients with PSP-P (Table 2). A comparison of the prevalence of NMSs between patients with PSP-RS and those with PSP-P revealed a higher prevalence of sexual dysfunction and a positive response to “Does the patient have altered interest in sex?” in patients with PSP-RS.
The severity of NMSs was significantly different for four questions on the NMSS: “Does the patient feel sad or depressed or has he/she reported such feelings?” (PSP-RS > PSP-P), “Does the patient dribble saliva during the day?” (PSP-RS > PSP-P), “Does the patient have altered interest in sex?” (PSP-RS > PSP-P), and “Does the patient report a change in ability to taste or smell?” (PSP-RS > PSP-P). In addition, the total domain score for sexual function was higher in patients with PSP-RS.
Partial correlations and linear regressions
Several significant correlations were observed between clinical scores and the NMSS domain subscores (Table 3). High positive correlations were observed between the domain of attention/memory (D3) and the HAM-D score. The sleep/fatigue domain (D2) showed a high positive correlation with total PSP-RS score, PSP-history score, PSP-bulbar score, and caregiver burden scale score. The gastrointestinal tract domain (D6) also showed a positive correlation with the total PSP-RS score, PSP history score, PSP bulbar score, and caregiver burden scale score. Finally, the sexual function domain (D8) correlated with the caregiver burden scale score. A high negative correlation was observed between the PDSS score and the gastrointestinal tract domain (D6), the total NMSS score, the sexual function domain and the caregiver burden score (r = 0.55, p < 0.01). No correlations were observed between NMSS scores and the duration of illness.
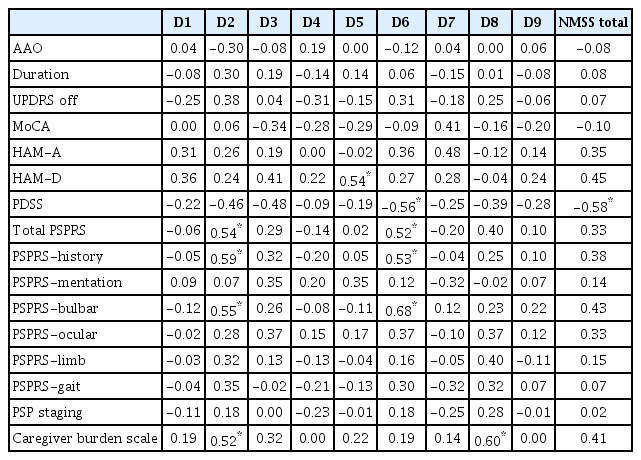
Partial correlation coefficients (r) between Non-Motor Symptom Scale domain scores and clinical variables in patients with progressive supranuclear palsy
Linear regressions performed on the above variables revealed the persistence of associations between all variables except for sleep/fatigue and caregiver burden scale score (Table 4).
DISCUSSION
NMSs significantly contribute to a worsening quality of life and increasing morbidity in patients with parkinsonian disorders. Although extensively explored and established in PD, the exact burden of NMSs in other parkinsonian disorders is uncertain. The present study aimed to explore the profile of NMSs in patients with PSP, to compare the NMSs between patients with PSP-Richardson syndrome and those with PSP-parkinsonism, and to identify the clinical correlates of NMSs in PSP. Additionally, we attempted to ascertain the impact of NMSs on caregiver burden. To the best of our knowledge, this is the largest study to explore the profile of NMSs in patients with PSP and to explore the differences in NMSs between PSP subtypes (Table 5) [7-11]. Although three previous studies [7,9,11] have used the NMSS in PSP, the evaluated sample sizes were relatively smaller (Radicati et al. [7]: 50, Ou et al. [9]: 27, Lee et al. [11]: 14). Other studies, which also have smaller sample sizes, either have utilized a different scale [14] or have primarily focused only on autonomic symptoms [8].
In the current study, all patients reported the presence of at least one NMS. The most prevalent NMSs were in the domains of sleep/fatigue, mood/cognition, and sexual function (Figure 1, Table 2). The least prevalent NMSs were in the domains of perceptual problems/hallucinations, followed by the CVS system, including falls. Although no differences in the total NMSS score were observed between patients with PSP-RS and those with PSP-P, patients in the PSP-RS subgroup reported higher severity for specific questions in the domains of mood/cognition, sexual function, and miscellaneous (Figure 1). In addition, a significantly higher prevalence of sexual dysfunction was observed in patients with PSP-RS. The following paragraphs will attempt to explain the basis for the observed NMSs in patients with PSP.
Sleep disturbances comprising daytime sleepiness, fatigue, difficulty falling or staying asleep and restless legs were the most prevalent NMSs in our cohort (Figure 1, Table 2), and “fatigue or lack of energy” was the most prevalent item in this domain (Table 2). This observation of sleep disturbances in PSP has been previously reported as the most prevalent NMS from questionnaire-based studies by Ou et al. [9] and Radicati et al. [7] and has also been reported in polysomnography (PSG)-based studies in patients with PSP [20]. Patients with PSP have been found to have disruptions in sleep architecture and in both sleep wake regulation mechanisms and circadian rhythm activity [21]. These abnormalities may be implicated in the preferential degeneration of pontine tegmental nuclei in PSP [21]. Additionally, the significant axial stiffness and difficulty turning in bed may also contribute to this NMS. This is supported by the significant correlations observed between the total PSPRS score and the sleep/fatigue domain score observed in the study, which suggests that an increase in disease severity tends to contribute to the worsening of symptoms in this domain (Table 3). In addition, we also observed correlations between the PSPRS history and bulbar subscores. These correlations may be due to the presence of a question related to sleep difficulty in the PSP-history subscore, and perhaps bulbar dysfunction may also contribute due to choking or coughing, which may occur due to difficulty swallowing saliva. These observations were supported by high p values obtained from the linear regression models (Table 3).
NMSs in the mood/cognition domain were the second most prevalent NMSs in this cohort, and a “lack of motivation” was the most reported item in this domain (Figure 1, Table 2). This domain also had the highest score on the NMSS. Neuropsychiatric abnormalities comprising cognitive dysfunction are predominantly frontal dysexecutive syndrome, and behavioral changes such as apathy, depression and impulsivity have been frequently reported in PSP [9,11,13,22,23]. These may be attributable to the degeneration of the medial frontal regions and insular cortex, which is observed in PSP [24].
Sexual dysfunction in patients with PSP has seldom been discussed or reported. In the present study, sexual function was the third most prevalent NMS domain, and “problems having sex” was the most commonly reported item in this domain (Figure 1, Table 3). The PSP-RS group reported a higher prevalence and severity than the PSP-P group. A reduction in libido has been previously reported in individuals with PSP [25], and previous NMSSbased studies have also reported sexual dysfunction [7,9]. This NMS in individuals with PSP may be attributable to the physical disability produced due to the disease process. Interestingly, this NMS was the only domain that significantly correlated with caregiver burden.
Gastrointestinal tract symptoms were also reported by a large proportion of patients in this cohort (Figure 1, Table 2). As expected, “dysphagia” was the most prevalent item in this domain (Table 2). Although frequently reported and a recognized symptom of PSP, it is inadequately explored. This is crucial since dysphagia plays a significant role in the development of aspiration pneumonia, which is a predominant cause of death in patients with PSP. In the present study, we observed significant correlations between the total PSPRS score and the domain score for gastrointestinal tract symptoms, suggesting that the severity of this NMS increases as the disease progresses. In addition to the overall PSPRS score, significant correlations were observed between the history and bulbar subscores. These results, especially the latter, suggest a direct association between NMSS scores and symptoms in patients with PSP. In addition, the high p values obtained from the linear regression models also support these findings (Table 3).
Attention/memory deficits in PSP have been frequently reported and were also observed in our cohort (Figure 1, Table 2). Patients reported maximal difficulty in “sustaining conversations during activities” (Table 2). Patients with PSP are known to have impaired attention and recall and an impairment in social functioning [13]. We observed a strong correlation between HAM-D scores and the attention/memory domain score. Memory disturbances are known to occur in depression, and it is possible that the mild depression observed in these patients contributes to the observed NMSs.
Urinary symptoms have rarely been studied in PSP, and few studies report the presence of this or clarify the etiology [26]. In the present study, “urinary urgency” was the most prevalent item in this domain (Figure 1, Table 2). In a previous study by Radicati et al. [7], the domain of urinary symptoms was the most prevalent NMS, and nocturia was the most frequent item. It is uncertain why this result varies between the present study and the abovementioned study. Although the cause of urinary dysfunction in patients with PSP is uncertain, detrusor sphincter dyssynergia, detrusor hyperreflexia and forebrain dysfunction may be implicated [27]. Urinary symptoms are commonly associated with multiple system atrophy, and the observation of urinary symptoms in patients with PSP was higher than anticipated. Patients with atypical parkinsonian features who complain of urinary symptoms early in the course of illness should also be evaluated for the possibility of PSP.
Patients with PSP also reported abnormalities in the domains of miscellaneous NMSs and CVS including falls, and perceptual problems/hallucinations (Figure 1, Table 2). Pain in individuals with PSP may be secondary to altered self-estimation of pain due to frontal cortical degeneration or associated with the degeneration of descending inhibitory control systems within the brainstem [28]. Recent changes in weight may be associated with dysphagia. Unlike in patients with PD, we did not observe a significant prevalence of hyposmia in patients with PSP [6]. Interestingly, a lower prevalence of abnormality in smell/taste was reported in the PSP-P subgroup than in the PSP-RS subgroup.
The domain of CVS including falls, was found to be the second least prevalent domain. Questions in this domain should be administered with care to aid in the differentiation of falls secondary to motor disturbance and falls due to autonomic dysfunction. Dizziness was the most prevalent item in the domain of CVS including falls (Table 3). Sympathetic and parasympathetic cardiac autonomic dysfunction has been reported in patients with PSP, and this may be implicated in the observed falls [8]. Finally, the least prevalent NMSs in patients with PSP were in the perceptual problems/hallucinations domain, wherein visual hallucinations were the most frequently reported (Table 1). Hallucinations are commonly reported in patients with PD and DLB and are less frequently reported in patients with PSP [29]. A mechanism similar to that of PD, i.e., denervation supersensitivity of mesolimbic and mesocortical dopaminergic receptors and other neurotransmitter imbalances, has been implicated for hallucinations in patients with PSP [29].
There are several limitations to this study. The NMSS is a scale primarily designed to assess NMSs in patients with PD and has not been validated in patients with PSP. However, in the absence of other validated scales for parkinsonian syndromes, we chose to utilize this scale to assess NMSs in patients with PSP. Furthermore, this scale was also recently used by Radicati et al. [7] in a study on NMSs in patients with PD and PSP. The present study was not a case-control study, and we did not compare the PSP group against healthy controls or individuals with other parkinsonian disorders. We did this with the objective of determining the impact and burden of NMSs in patients with PSP and their caregivers. Despite the use of consecutive sampling to collect data, there was a significant difference in gender in the present cohort, which may be a confounding factor.
In conclusion, all patients with progressive supranuclear palsy reported a minimum of two NMSs, with the domains of sleep, mood and sexual function being the most commonly affected. The severity of these NMSs was unrelated to the duration of illness. NMSs contribute significantly to disease morbidity, and adequate attention should be paid to the identification and treatment of these symptoms.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.
Author Contributions
Conceptualisation: all authors. Data curation: Sudhakar Pushpa Chaithra, Shweta Prasad, Vikram Venkappayya Holla, and Albert Stezin. Formal analysis: Sudhakar Pushpa Chaithra, Shweta Prasad, Vikram Venkappayya Holla, and Albert Stezin. Supervision: Nitish Kamble, Ravi Yadav, and Pramod Kumar Pal. Writing—original draft: Sudhakar Pushpa Chaithra, Shweta Prasad, Vikram Venkappayya Holla, and Albert Stezin. Writing—review & editing: Nitish Kamble, Ravi Yadav, and Pramod Kumar Pal.
Acknowledgements
None.




