TNR Gene Mutation in Familial Parkinson’s Disease: Possible Implications for Essential Tremor
Article information
Dear Editor,
Farlow et al. [1] reported in 2016 new rare tyrosine kinase non-receptor 2 and tenascin R (TNK2 and TNR, respectively) variants associated with the development of Parkinson’s disease (PD). No detailed clinical information about such patients or other reports is available thus far. We report a subject with PD and two of his sons with essential tremor (ET) harboring a TNR gene mutation.
A 77-year-old right-handed Mexican man developed right arm resting tremor, rigidity and bradykinesia 6 years previously. Constipation, nocturia, urinary urgency and incontinence, fatigue, excessive daytime sleepiness, depression, anxiety, hypoprosexia and a dysexecutive syndrome began 2 years prior. There were no other signs or symptoms. Levodopa/benserazide 100/25 mg QID improved most of his signs and symptoms. The subject’s mother and two of his siblings were diagnosed with PD as well. There was no consanguinity. The subject had two sons and two daughters; one son and one daughter had tremor and were diagnosed with ET in late childhood (Figure 1A). Neurological examination revealed a Montreal Cognitive Assessment score of 27 points, facial hypomimia, monotone voice, resting, postural and kinetic tremor of the right arm, ipsilateral bradykinesia and rigidity. Diminished right-arm swinging during walking was also noticed. Postural reflexes were unaffected. In the proband’s daughter and son, neurological examination showed slight postural and kinetic tremor in both hands, as well as writing anomalies (Figure 1B and C).
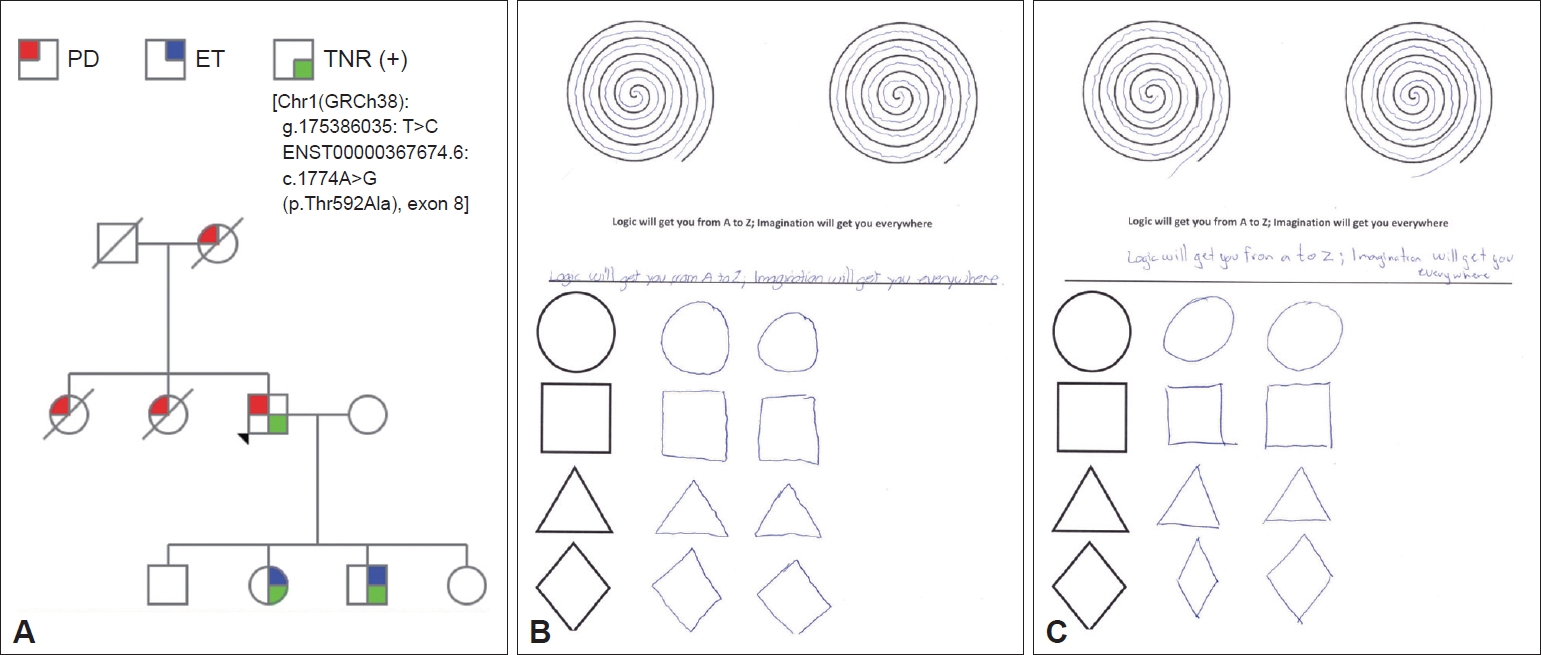
Genetic and clinical features. A: Family pedigree. Further information about the clinical features of the proband’s mother and sisters is missing. The index case developed PD symptoms at the age of 71. The proband’s daughter developed ET at the age of 13 and the proband’s son at the age of 10. B: Writing test of the proband’s daughter with ET. C: Writing test of proband’s son with ET. PD: Parkinson’s disease, ET: essential tremor, TNR: tenascin R.
Whole-exome sequencing as well as an extensive genetic panel for neurological diseases was performed for all live family members. A likely pathogenic heterozygous mutation in the TNR gene [Chr1(GRCh38): g.175386035: T>C ENST00000367674.6:c.1774A>G (p.Thr592Ala), exon 8] was detected in the index case and in his son and daughter diagnosed with ET.
Neurological assessment of the son and daughter with a positive genetic test showed only bilateral action tremor affecting both hands, fulfilling the criteria for ET.
The tenascin family of glycoproteins comprises four types in vertebrates, namely, tenascin-C, -R, -W and -X, of which tenascin-C (TNC) and TNR are particularly important for central nervous system physiology [2]. Moreover, tenascins appear to be critical for normal tyrosine hydroxylase mRNA expression and development of the basal ganglia [3]. TNR is exclusively expressed in the nervous system, mainly in oligodendrocytes but also in other neuronal subtypes, such as inhibitory interneurons in the striatum, hippocampus and cerebellum, and Schwann cells in the peripheral nervous system [2].
Farlow et al. [1] reported for the first time five exonic variants of the TNR gene associated with PD susceptibility, one of which (c.1774A>G, p.Thr592Ala) was found in the present family. All TNR mutations were described as heterozygous, consistent with dominant inheritance [1]. Our index case experienced late-onset PD with a benign course to date. No previous history of tremor was reported, though one of his sons and one of his daughters developed ET in childhood. Except for age of onset (50.1 ± 15.7 years), the full clinical phenotype of subjects described by Farlow et al. [1] still needs to be reported in terms of progression and long-term follow-up. Larger series and longer follow-up periods may help elucidate these and other aspects of the TNR-related phenotype.
Given the recent evidence highlighting TNR mutations as a risk for PD, the spectrum of possible mechanisms involved in its pathogenesis widens, as do research avenues for disease-modifying treatments. In a model of Huntington disease, Hargus et al. [4] transfected protein-labeled murine embryonic stem cells (ESCs) overexpressing TNR in striatal GABAergic neurons. In comparison with sham-transfected control cells, TNR-overexpressing ESCs showed enhanced differentiation into neurons in vitro, reduced migration in vitro and in vivo, increased generation of GABAergic neurons and decreased numbers of astrocytes after transplantation, without significant effects on locomotor functions [4]. TNC also has proven potential treatment properties in PD. In a rat model investigating the methods to deter anoikisinduced apoptosis within cell cultures and grafts of primary mesencephalic dopaminergic neurons, researchers found that pretreatment with TNC was beneficial to prevent cell death in this scenario [3].
TNR gene mutations have not been previously associated with ET, which is supposedly a cerebellar condition. Interestingly, TNR associated with Purkinje cell bodies and their dendrites in the molecular layer of the cerebellum bears N-linked oligosaccharides terminating with beta1,4-linked GalNAc-4-SO4, whereas TNR in other regions of the cerebellum does not show this modification. Expression of this unique sulfated carbohydrate structure is also temporally regulated, increasing throughout cerebellar development. The spatially and temporally regulated addition of this unique sulfated carbohydrate to TNR may modulate its adhesive/anti-adhesive or other biological properties in vivo [5]. TNR-deficient mice exhibit impaired motor coordination deficits, including poor performance in the rotarod test, indicating a deficit in cerebellar functions. Interestingly, basket and stellate cells, inhibitory interneurons of the cerebellar cortex, use GABA as neurotransmitter and express TNR. The deficits of TNR-deficient mice with regard to motor coordination may result from alterations of the cerebellar GABAergic system [6].
The relationship between ET and PD is still under scrutiny, but some genes have been shown to produce a different phenotype in the same family (e.g., HTRA2) [7], thus indicating that the isolated action tremor of some family members might represent a PD-related endophenotype. However, the association in this case report may also be a coincidence, as ET is a common entity.
This is the first clinical description of PD associated with a TNR gene mutation after the original description by Farlow et al. [1]. We hypothesize that ET subjects may carry this gene variant. Future studies should further explore Farlow et al.’s hypothesis that variants of intermediate penetrance remain a major contributor to PD heritability. Future studies should also confirm an association between ET and TNR mutations. In this respect, experimental designs focusing on intrafamilial and interfamilial heterogeneity will be of particular relevance.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.
Author Contributions
Conceptualization: Michel Sáenz-Farret, Carlos Zúñiga-Ramírez. Supervision: Renato Puppi Munhoz, Alfonso Fasano, Carlos Zúñiga-Ramírez. Writing—original draft: all authors. Writing—review & editing: all authors.
Acknowledgements
We would like to acknowledge Carlos Piña-Avilés, MD for providing assistance in the preparation of this manuscript.
