Electrophysiologic Evaluation of Psychogenic Movement Disorders
Article information
Abstract
Psychogenic movement disorders (PMD) are a group of disorders which are in the border zone between neurology and psychiatry. All necessary laboratory investigations should be done to rule out an underlying organic disorder. While clinical acumen of a trained movement disorder specialist may be sufficient to diagnose most PMD, there are clinical situations where electrophysiological tests are required either to rule out an organic movement disorder or even diagnose a PMD. Current electrophysiological test are most useful for tremor, followed by jerks and least for spasms or dystonia. Commonly used electrophysiologic tests include multichannel surface electromyography (EMG), accelerometry, electroencephalography time locked with EMG, premovement potential (Bereitschaftspotential), and somatosensory evoked potentials. Psychogenic tremor is a low frequency tremor with variable frequency and duration of EMG bursts, entrainable, has a high coherence with voluntary movements, and presence of coactivation sign. Patients with psychogenic jerks have well organized triphasic pattern of activation of agonist and antagonist muscles. The jerks are associated with EMG bursts of long duration (usually > 70 ms), long and variable latencies in stimulus induced jerks, absence of craniocaudal pattern of muscle recruitment in apparent startle response, and often a Breitschaftspotential (premovement potential) precedes the jerk. Electrophysiological characterization of psychogenic dystonia is difficult and the tests are usually performed to rule out organic dystonia with characteristic findings. Finally, caution should be exerted in interpreting the electrophysiological tests as both false positive and false negative diagnosis of PMD may still occur.
Psychogenic Movement Disorders (PMD) are one of the most challenging neuropsychiatric disorders to both the Neurologist and the Psychiatrist which have baffled the specialists of both these disciplines, time and again, over more than a century. These disorders cannot be fully accounted for by any known organic syndrome and which appear, as based on available clinical evidence, to have significant psychological and or psychiatric contributions. Elegant descriptions are available in writings of Charcot,1 Gowers,2 and Head.3
The psychogenic neurological disorders include psychogenic hemiplegia, paraplegia, blindness, seizures, pain syndromes, and a variety of movement disorders PMD. The latter include hyperkinetic syndromes of tremor, jerks, and spasms (dystonias), gait disorders, and less commonly a hypokinetic extrapyramidal syndrome mimicking parkinsonism. PMD has also been sub classified into dystonic and non-dystonic.4 While clinical acumen by trained neurologist is often sufficient to diagnose some PMDs, there are clinical situations when clinical characteristics may be inconclusive to make a firm diagnosis of PMD, which is required for management of the patient. There are no specific laboratory investigations to diagnose PMD and appropriate investigations need to be carried out to rule out an organic basis of these neurological disorders. However, over the past two decades, several investigators have reported usefulness of electrophysiological tests to differentiate between true and psychogenic movement disorders. This article will review the role of electrophysiology in diagnosis of PMD.
The exact prevalence or incidence of PMD is unknown. Small cohorts of patients and inadequate follow up preclude adequate information on the epidemiology of PMD. The prevalence of psychogenic neurological disorders have been reported to be 1–9% among patients attending Neurology clinics.5–7 Among the patients attending specialty clinics of movement disorders, the prevalence has been reported to vary from 2.1% (4) to 3.3% (8). The prevalence of PMD is higher in women compared to that in men (−3 : 2).4,8 Age of the patients vary from 17–83 years with a mean age of 50.8 The age of onset of PMD is quite variable, and has been reported to be 8–58 years.4
Tremor is the most commonly observed PMD8,9 though in a large series10 dystonia was found to be commonest. On the contrary, Monday and Jankovic11 reported psychogenic myoclonus to be the commonest PMD (20.2%) of the 89 PMD patients seen in the movement disorder clinic at Baylor College of Medicine, Houston, Texas, USA, from 1981 to 1991. The other PMDs are by gait disturbances, parkinsonism, stiff person syndrome, tics, belpharospasm, hemifacial spasm and other facial movements, paroxysmal dyskinesias or shaking, tics, chorea and undifferentiated movements. In addition to a PMD, a patient may have another PMD (e.g., tremor and dystonia), an organic movement disorder, or a psychogenic neurological disorder other than movement disorder (e.g. hysterical paralysis, blindness, or pain). A combination of PMD and organic movement disorder has been reported in 10–25% of cases8,12 and usually the organic disorder precedes the PMD by years.
Coexistent psychiatric diagnosis in the form of anxiety (38.%) and major depression are common (19.1%) in patients with PMD, and they also have a higher chance of other life-time psychiatric diagnosis, including adjustment disorder, schizoaffective disorder, bipolar disorder, and alcohol or sedative abuse.13 In general, patients with PMD generally fall into one of the two broad categories of psychiatric dysfunctions:14 1) symptom production not under conscious voluntary control which include conversion disorder and somatization disorder (Hysteria or Briquet’s syndrome); and 2) symptom production under voluntary control which include patients either suffering from factitious disorder or malingering.
Clinical characteristics of PMD
A detailed clinical history with separate interviews of the patient and the caregiver, meticulous neurological examination, and prolonged observation of the patient over several sessions usually provide sufficient clues to make a diagnosis of PMD. Patients with PMD usually have acute or subacute onset of symptoms with maximum severity at the onset and thereafter a static course. In a large majority of patients a clear precipitating factor, often in the form of psychosocial stress, is present and there may be associated past or present psychiatric illness. The PMD may be paroxysmal, mimicking organic paroxysmal movement disorders. Clinical features of PMD include: 1) increase or become elaborate when examination is focused on affected part, 2) decrease or resolve when not the clear focus of attention or enquiry and during tests requiring concentration or other tasks, 3) triggered or relieved with unusual nonphysiological interventions (such as trigger points on the body, tuning fork), 4) deliberate slowness of movements, 5) rhythmical and often violent shaking, 6) changing characteristics of movements -severity, frequency, type, and distribution, 7) entrainment (see below), 8) selective disability, 9) demonstration of fatigue, 10) presence of multiple movement disorders, 11) presence of bizarre movements which are difficult to classify, 12) excessive startle response, 13) paroxysmal movement disorders, and 14) bizarre gait
Psychogenic tremor
Psychogenic tremor most often involves the right hand (84%), followed by legs (28%), generalized (20%), left arm and head (8% each).15 Tremor of voice, face, tongue, and fingers is distinctly uncommon. Patient with psychogenic tremor may have a selective disability, e.g., normal writing but tremulousness on drawing a spiral. Suggestion and placebo administration can be used diagnostically to exacerbate or relieve tremor.
A patient with psychogenic tremor of one limb, when asked to perform rapid or slow movements with his “unaffected” limb, may fail to continue with the original frequency of the “abnormal” movement of the “affected” limb. The frequency of tremor of the affected limb may be entrained by the new frequency of the unaffected limb (entrainability of tremor). This clinical observation can be confirmed by coherence analysis, described below.
Another feature which is often characteristic of psychogenic tremor is the inability of the patient to maintain the same frequency or pattern of abnormal movements when observed over prolonged periods. A flexion-extension movement of the fingers may change to a pronation-supination movement of the forearm. While a patient of organic tremor can maintain the same frequency of tremor even on performing mental tasks such as serial-7 subtraction or when engaged in conversation, a patient will psychogenic tremor usually fails to maintain the same frequency. This phenomenon, called “distractibility” was present in 86–100% of patients with psychogenic tremor15,16 and may sometimes need electrophysiological evaluation for documentation.
“Coactivation sign” described to be a characteristic feature of psychogenic tremor, consists of presence of voluntary coactivation of agonist and antagonist muscles of the respective joint with overlying rhythmic trembling.15 This sign can be elicited by palpating the muscles when testing for rigidity. In the tremulous hand, there is active resistance against passive, arrhythmic movements performed by the examiner about the involved joint in opposite directions (e.g., flexion and extension of wrist). There is fluctuation of the tone with reduction or increase in the tremor. Finally there may be a temporary but complete normalization of the tone with disappearance of tremor. This co-activation sign can be electrophysiologically documented (see below).
Psychogenic dystonia
Among the patients diagnosed as dystonia, the prevalence of psychogenic dystonia may range from 2.2% to 4.6% depending on the criteria used (documented, clinically definite, probable or possible psychogenic dystonia).10,17 It is important to note that in 25–52% of cases organic dystonia may be misdiagnosed as psychogenic dystonia.16,18–21 The reasons for this misdiagnosis are several common characteristics between organic and psychogenic dystonias, such as features of 1) varied nature of abnormal movements, alone or in combination, or co-occurrence of organic and psychogenic movements in the same patient 2) spontaneous remissions (in organic dystonia up to 20% cases, especially cervical dystonia), 3) task specificity, 4) paroxysmal course, 5) absence of other neurological deficits and normal investigations (e.g., in idiopathic torsion dystonia), 6) temporary relief by “sensory tricks” (geste antagoniste) (though characteristic of organic dystonia, has been also reported in psychogenic dystonia,22 7) relief or reduction by relaxation and hypnosis, 8) even minor trauma (head/neck or peripheral) may precede onset of true dystonia. In addition, patients with psychogenic dystonia may have relief of dystonia by trigger point pressure or injection (e.g., of saline).
Psychogenic myoclonus
A diagnosis of psychogenic myoclonus is made when patients have jerks inconsistent or incongruous with typical myoclonus and have clinical characteristics of PMD described previously, such as diminished movement with distraction, periods of spontaneous remission or remissions following suggestion and placebo, presence of other psychogenic symptomatology, and evidence of psychopathology by testing or by past psychiatric history.11 The distribution of psychogenic myoclonus has been reported to be predominantly segmental or generalized and less often focal.11 As with other PMDs, women are more often affected and patients may have additional PMDs such as gait, tremor and dystonia. Patients with psychogenic myoclonus involving the whole body and precipitated by stimulus, habituate with repeated stimuli, like normal startle response.23 However, psychogenic myoclonus may mimic closely propriospinal myoclonus and in such situation electrophysiological evaluation may be helpful (see below).
Psychogenic parkinsonism
Parkinsonism as a primary psychogenic manifestation is rare. In the series by Lang et al.24 the mean age of presentation was 47 years and 71% noted symptoms after work-related injury or motor vehicle accident. Majority (57%) had bilateral symptoms and the nature of tremor, rigidity, bradykinesia differed from idiopathic Parkinson’s disease. Apart from the characteristics of psychogenic tremor described above, the “rest” tremor may fail to change with posture or action. Early maximum disability with a static course, voluntary resistance (often reducing when distracted) rather than a true rigidity, absence of micrographia, normal facial expression and voice, arms held stiffly at the sides while walking, and extreme or bizarre response to minimal displacement on “pull test” are other characteristic features of psychogenic parkinsonism.24
Red flags in diagnosis of psychogenic tremor
Appropriate caution should be exerted in labeling an organic tremor syndrome as psychogenic tremor. Psychogenic tremor may coexist with organic movement disorders including tremor. Tremor following vascular strokes and trauma may have abrupt onset, and that secondary to exposure to toxins or drugs may have fluctuation or spontaneous remissions, simulating psychogenic tremor. “Rubral” or midbrain tremor following demyelination, trauma, or that in Wilson’s disease can have a combination of coarse rest and action (postural or kinetic) tremor can be sometimes mistaken for psychogenic tremor. Finally a feeling of tremulousness in legs when standing, inability to stand, but ability to walk normally after few steps can be a manifestation of orthostatic tremor, which may be overlooked.
Diagnostic Criteria of PMD
There are no diagnostic laboratory criteria for PMD. A valid positive criteria of PMD based on clinical examination is rare, and currently the diagnosis is based on negative criteria, viz. absence of symptoms indicative of an underlying organic disease.
Fahn and Williams10 and Fahn4 categorized patients into four levels of certainty as to the likelihood of their having a PMD. The degrees of certainty are documented PMD, clinically established PMD, probable PMD, and possible PMD. With availability of advanced electrophysiological techniques to evaluate certain PMDs, Brown and Thompson25 proposed a combined clinical and electrophysiological classification of PMD. The classification included definite, probable and possible PMD, with supporting electrophysiological evidence of PMD in the first two categories.
Electrophysiological Evaluation of Movement Disorders
Electrophysiological evaluation of movement disorders help to identify and characterize a variety of abnormal movements which include tremor, myoclonus, dystonia, tics, asterexis, ataxias, etc. It also helps to characterize mixed movement disorders or clinically bizarre movements. In certain cases, such as myoclonus or tremor, advanced techniques of electrophysiology may also help to precisely locate the origin of these movements in the central nervous system.
With advances in electrophysiologic techniques there has been greater understanding of the pathophysiology of movement disorders, identification of characteristic physiological changes of many of these disorders, and recognizing patterns of movement that could be under voluntary control.25
Electrophysiological evaluation of different types of PMD is well established. Currently available electrophysiological tests are most useful for tremor, and to some extent for myoclonus, and least for dystonia. The commonly used tests are multichannel surface electromyographic (EMG) recording, accelerometry, electroencephalography (EEG) time locked with EMG, premovement potential (Bereitschaftspotential), and somatosensory evoked potentials. Other tests which may be of value include long-loop responses, H-reflex recovery curve, and transcranial magnetic stimulation (TMS).
Evaluation of psychogenic tremor
The basic electrophysiological evaluation of tremor usually involves recording the actual movement of body parts using an accelerometer and the EMG activity from the involved muscles using surface electrodes. Multi-channel surface electromyography and at least two accelerometers are required for evaluation of psychogenic tremor. Clinical examination and prolonged observation usually help the examiner to identify the muscles which are “tremulous” and require to be studied. The patient is comfortably seated in a chair and explained about the test and the different maneuvers which need to be performed while tremor recording. It is recommended to acquire EMG and accelerometry data for sufficient lengths of time (100 seconds to even few minutes) for each condition (described below).
On the affected limb, surface electrodes are placed in a tendon-belly arrangement to record EMG activities from the antagonistic muscles. The actual movement of the body part can be recorded by a lightweight piezoresistive accelerometer strapped over body parts involved in tremor. Single axis accelerometers are commonly used, but triaxial ones are available to record the movements in three axes (vertical, horizontal, and antero-posterior). On the unaffected side, two-channel surface EMG, examining the flexors and extensors of the wrist or fingers may be sufficient along with one single-axis accelerometer placed over the moving part. This will help to capture the voluntary movement which the patient will be required to do to study the entrainability and, distractibility of the tremor of the affected side and coherence.
Protocol and analysis
The protocol for evaluation of psychogenic tremor is given in Table 1. Tremor is recorded in different conditions to document changes of the characteristics of tremor with posture, voluntary movements of unaffected body part, distraction, weight-loading, peripheral and central stimulation, etc. After acquisition of EMG and accelerometric data, further analysis is done to characterize the tremor (Table 2): a) determine the frequency and power spectrum of the tremor, b) pattern of EMG bursts, c) modulation of frequency and amplitude of tremor by mental tasks, and movement of other parts of body, and d) determine whether tremor with different frequencies coexist.
Pattern of EMG bursts
The majority of patients with ET (type A), cerebellar, and enhanced physiological tremor have a synchronous pattern of muscle activation while an alternating activation pattern is seen in patients with PD rest tremor, rubral tremor, some patients with ET (type B). In psychogenic tremor usually an alternating pattern is seen. It should be remembered that in 10–15% of PD patients, a synchronous pattern can be found during action but a reciprocal alternating pattern during rest and this feature should not point towards a non-organic basis. In addition, it has also been observed in the same patient both patterns of muscle activity can occur.
Duration of EMG bursts
The duration of EMG bursts are often helpful to differentiate between organic and psychogenic tremors. The EMG bursts in psychogenic tremors is usually > 70–80 ms. However, it should be noted that in organic tremors with EMG recorded from larger muscles as well as dystonic tremor can have longer duration of EMG bursts. In psychogenic tremor, duration of EMG bursts are often varying (Figure 1). However, this feature can also be present in dystonic tremor.
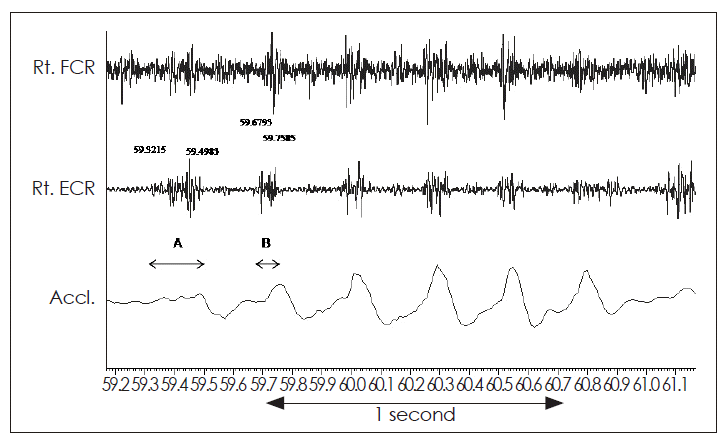
Tremorogram from the right hand of a patient with psychogenic tremor of the right hand. Surface electromyographic recording from the right flexor carpi radialis (Rt. FCR) and right extensor carpi radialis (Rt. ECR). The accelerometric recording from the wrist is shown in lowest trace. Note the marked variation in the duration of EMG bursts recorded from Rt. ECR, which varied from 176.8 ms (A) to 79.2 ms (B).
Coactivation sign
This sign is often observed in patients with a psychogenic tremor at the onset of tremor. Electrophysiologically, there is a tonic coactivation phase of the wrist flexor and extensor muscles -300 ms before the reciprocal alternating tremor bursts develop.15
Tremor amplitude
The amplitude of tremor is variable and is not of much diagnostic significance to differentiate between organic and psychogenic tremor. In psychogenic tremor apart from a change of frequency with distraction or suggestion, there may be a change is amplitude also. However, amplitude of tremor is very variable in organic tremors. In contrast to organic tremors, in psychogenic tremor there is an increase in tremor amplitude with peripheral loading.
Tremor frequency
Analysis of the frequency of tremor is probably the most important step to differentiate between organic and functional tremors. Based on the frequency, tremor is classified as low, medium and high frequency. Though the frequency of tremor can be determined by counting the number of EMG bursts over a given length of time, power spectrum analysis is preferable. The accelerometer recording and the rectified and digitally filtered EMG (below 50–100 Hz to exclude movements artifacts) of each muscle is subjected to Fast Fourier Transformation to extract the peak frequency and the total power in the frequency range between 2 and 30 Hz.26 Tremors > 11 Hz are rare and are likely to be pathologic (e.g., in orthostatic tremor), tremor < 6 Hz are almost always pathologic.26
The characteristics of psychogenic tremor are given in Table 3. These include 1) usually a low frequency tremor which is < 11 Hz, 2) varying frequency of tremor (Figure 2), 3) change of frequency with distraction (usually on a mental-arithmetic task), 4) a dissipated frequency spectrum of tremor rather than a peak frequency, 5) positive entrainment of tremor (when asked to tap out a beat with the limb contralateral to the tremulous limb, tremor in the latter either dissipates or shifts to the frequency of tapping), 6) high coherence between the “involuntary” and voluntary movements (see below), and 7) and absence of a frequency dissociation (simultaneous occurrence in separate muscles groups of tremors with different frequencies).
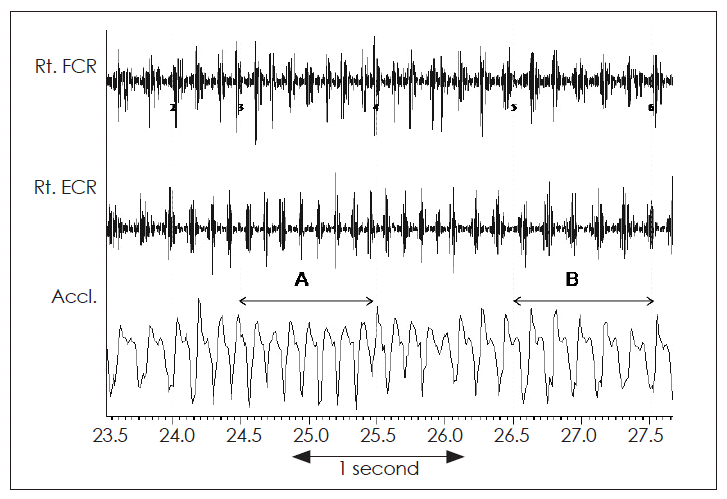
Tremorogram from the right hand of a patient with psychogenic tremor of the right hand. Surface electromyographic recording from the right flexor carpi radialis (Rt. FCR) and right extensor carpi radialis (Rt. ECR). The accelerometric recording from the wrist is shown in lowest trace. Significant variation in the tremor frequency is seen from 8 Hz (A) to 5.5 Hz (B) even without any distraction.
The clinical findings of variation of frequency and amplitude of tremor with distraction, coactivation of antagonist muscles just prior to onset of tremor, entrainment of tremor frequency by the frequency of voluntary movement of unaffected limb, can be confirmed by electrophysiological tests and may be even helpful to diagnose non-organic origin of a tremor, when clinical methods fail. The frequency of psychogenic tremor can vary spontaneously without any distraction (Figure 2). When a patient is asked to do voluntarily rhythmic movements with the unaffected limb or body part, the tremor of the “affected” limb or body part may momentarily stop (Figure 3), and later its frequency may get dissipated (Figure 4) or entrained by the frequency of the voluntary movement of the unaffected limb. In a patient with organic tremor (e.g., essential tremor), the tremor frequency usually will remain unchanged when the patient is asked to do a mental task, such as counting 100–7 serially backwards. However in a patient with psychogenic tremor, the frequency of tremor changes and the tremor gets dissipated.
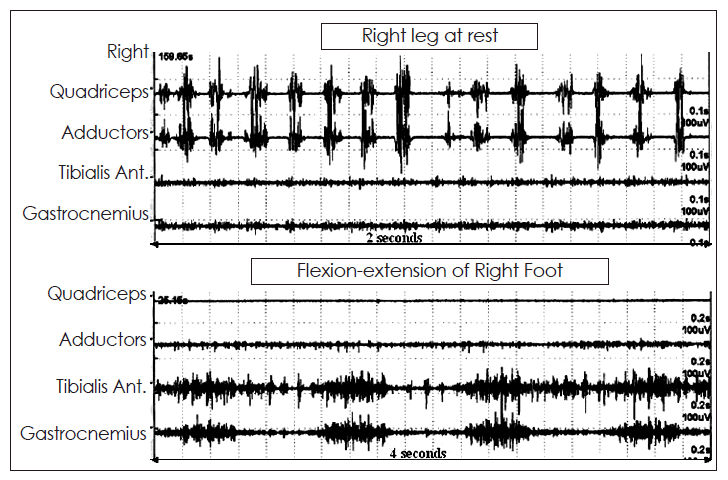
Tremorogram of a patient with jerky tremulous movements of the right thigh. The surface EMG recordings are from the quadriceps and adductor muscles of the thigh and tibialis anterior and gastrocnemius muscles of the leg. In the upper trace, the recording is at rest when the patient was asked to relax without any voluntary movements of the leg. In the lower trace, the patient was asked to move the toes up and down. It can be seen that there was an irregular tremor of approximately 7.5 Hz with variable duration of the EMG bursts as well as inter-burst intervals. When the patient was asked to voluntarily move the toes, this tremor of the thigh totally disappeared, suggesting distractibility and modulation, thereby supporting a non-organic basis of these movements.
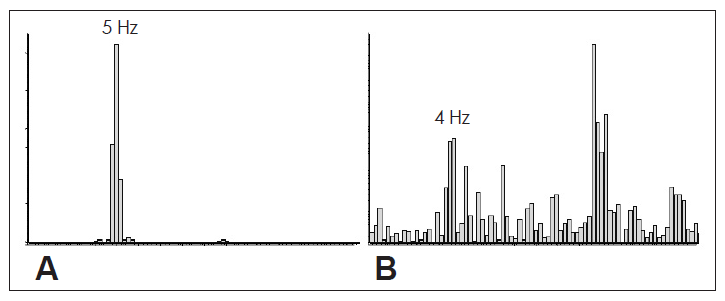
Power spectra of the frequency of tremor derived from accelerometric recordings from right hand kept at rest in a patient with psychogenic tremor of right hand. Figure 4A shows a well defined peak with the tremor frequency of 5 Hz. When the patient is asked to tap on the table with the left hand, the frequency of tremor becomes irregular, which is reflected in the power spectrum analysis of the accelerometric recording (B) which shows a dissipated or broadening spectrum with no well defined peak. This shows the distractibility of psychogenic tremor.
Electrophysiological tests often are used to support a diagnosis of organic tremor. For example, demonstration of a high frequency (13–18 Hz) tremor in muscles of lower limbs in a patient with “unexplained” gait disorder mimicking psychogenic gait might support a diagnosis of orthostatic tremor.
Coherence analysis
Coherence is the quantification of the linear association of two processes. If occurrence of one process can always be predicted by the second process coherence is said to be maximum (=1) and its values range from 0 (no coherence) to 1 (total coherence). Coherence is analyzed by using the spectral frequencies of EMG activities of two muscles or accelerometer recordings of movements of two body parts. Typically the patient is asked to voluntarily flex and extend the fingers or wrist of the unaffected hand, first at a slow rate and then at a faster rate. The surface EMG of the “tremorogenic” muscles of the affected hand (or any other body part affected by tremor) and of the muscles performing the above voluntary activities are simultaneously recorded. In addition, the movements of the two body parts are also recorded simultaneously by accelerometers. The concept underlying the coherence test is that it is very difficult to voluntarily tap simultaneously with two hands at slightly different frequencies.27,28 The two hands instead become entrained together to the same or to harmonically related frequencies. On the contrary, it is possible to tap simultaneously with an organic tremor that is generated by a separate pathological rhythm generator.29
In psychogenic tremor, there is a high coherence between the frequency spectra of voluntary activity and the “involuntary tremor” (Figure 5). McAuley and Rothwell29 using coherence entrainment electrophysiological test to study 25 patients with suspected psychogenic tremor or dystonic tremor and 10 normal subjects attempting to tap two independent voluntary oscillations. Among the patients, 6 were clinically definite dystonic tremor, 5 of probable dystonic tremor, 2 of classic essential tremor, 5 of clinically definite psychogenic tremor, 3 of probable psychogenic tremor and 4 uncertain cases. On comparing these clinical diagnoses with those reached by a coherence entrainment test subsequently carried out on each patient, there was 100% concordance in both clinically definite and clinically probable patients. In uncertain cases, the follow up clinical diagnosis also corroborated with the coherence entrainment diagnosis. No normal subjects were able to “mimic” organic tremor. The authors concluded that coherence entrainment test is a sensitive and specific means of distinguishing psychogenic tremor from dystonic and other organic tremors.

The figure demonstrates the phenomenon of coherence in a patient with psychogenic tremor of right hand. Simultaneous EMG and accelerometry were performed on both hands. The right hand was at rest (A and D) and the patient did fast tapping (B) and later slow tapping (E) on the table with the index finger of left hand. During fast tapping with left index finger, the tapping frequency as well as the right hand tremor frequency had identical peaks at 4.3 Hz with harmonics at 8.6 to 8.7 Hz. Coherence was seen at 4.5 Hz to 5.2 Hz. During slow tapping with left index finger (at around 2.3 to 2.6 Hz) the right hand tremor at rest was at 3.8 Hz with a harmonic at 7.8 Hz. The coherence between the movements of the two sides was at 2.3 Hz. This coherence test proves the entrainment of the tremor of right hand with the tapping frequency of left hand, thereby suggesting a psychogenic tremor.
Evaluation of psychogenic jerks
Electrophysiological tests for evaluation of jerks include surface EMG, EEG, premovement potentials, somatosensory evoked potentials, and long-latency or long-loop responses. The tests are selected depending on the type of jerk (spontaneous, reflex, or psychogenic) and the clinical suspicion (myoclonus, chorea, tics, voluntary, etc.).
Surface EMG
As in evaluation of tremor, surface EMG is recorded from the agonist and antagonist muscles of a joint directly involved in the jerk and the movement is recorded by transducer strapped over the moving body part.
In addition to the apparently affected muscles, EMG of other muscles, especially the cranial musculature (such as orbicularis oculi, sternocleidomastoids), and the proximal and distal muscles of other limbs not clinically involved in producing the jerks may often need evaluation. This will help to identify a craniocaudal progression of EMG activity bilaterally in startle reflex and to differentiate between cortical, brainstem and spinal myoclonus.
The protocol in evaluation of psychogenic jerks include recording the EMG activity at rest and during action. Psychogenic jerks often involve spinal musculature, especially while standing and walking or performing a specific task. Effort should be made to record EMG activities during these activities. Finally muscle activities should be recorded following unexpected stimuli such as electrical stimuli, sound, touch, etc. The external stimulus initiates a sweep from which the latency of the reflex EMG response can be calculated.
EMG is analyzed to determine the durations of EMG bursts, pattern of activation between the agonists and antagonists muscles of a joint, temporal relationship between activation of different muscles, latency between an external stimulus and onset of EMG burst, the relationship between movement of the body part and EMG activity, and, the habituation of reflex activity with repeated stimuli.
Electroencephalograph
EEG helps to correlate the cortical activity with muscle activity and may help in identifying the origin of myoclonic jerks and differentiating organic from psychogenic jerks. In addition to the routine EEG recording to detect spike or sharp wave discharges and determine their temporal relationship with the jerks, jerk-locked back averaging EEG may detect cortical discharges that precede cortical myoclonus.30
Premovement or bereitschaftspotential
With few exceptions, the premovement or Bereitschaftspotential (BP) is one of the most important electrophysiologic tools used to differentiate psychogenic from organic jerks. BP likely reflects the cortical activity prior to voluntary movement and thus represents movement preparation. It is a slow positive potential beginning around one second prior to movement and maximum over the vertex31 and is measured using back-averaging epochs of EEG preceding the EMG accompanying spontaneous jerks. This can be compared to BP recorded from voluntary, self-paced movements. For detecting such a potential several trials need to be averaged (at least 40 trials) to improve the signal-to-noise ratio, and it is unreliable if the movements occur more than every 2 seconds25 A jerk preceded by a BP is likely to be of voluntary origin.
Somatosensory evoked potentials
Somatosensory evoked potentials (SSEP) is usually recorded from stimulation of median nerve at the wrist and posterior tibial nerve at the ankle. Patients with cortical myoclonus may have enlarged SSEP.
Long-latency, long-loop response
Long-loop response (LLR) are motor responses to somesthetic stimuli occurring later than the H reflex. It is a reflex muscle response to a peripheral motor nerve stimulation coordinated at the level of the sensorimotor cortex. It is elicited by stimulating median nerve at the wrist and recorded from the partially contracted abductor pollicis brevis muscle. Simultaneous EMG is also recorded from the proximal muscles of the same limb and also from muscles of the non-stimulated hand. In addition, simultaneous median SSEP is recorded from the scalp. In certain types of myoclonus, especially cortical reflex myoclonus, a markedly enhanced long-latency reflex is usually recorded from the thenar muscle at a latency of around 45 ms after stimulation of the median nerve at the wrist,32 which corresponds to the C reflex named by Sutton and Mayer.33 In some patients, the enhanced C reflexes can also be recorded from more proximal muscles of the stimulated upper extremity with shorter latency and even from the opposite (nonstimulated) hand muscle.
Comparison of electrophysiological characteristics of organic and psychogenic jerks
Electrophysiological evaluation helps to characterize myoclonus and differentiate organic from psychogenic jerks.
Organic jerks
In most types of jerks, the movement of the body part is associated with EMG activity of the corresponding muscle except in asterixis or negative myoclonus where the movement is associated with silence in muscle activity. Organic jerks are most often characterized by bursts of EMG activity < 70 ms (Figure 5A) and co-contraction of agonist and antagonist muscle pairs.25 It is useful to determine the temporal relationship of a cortical activity with the EMG burst. Three patterns of temporal relationship can be observed: a spike or sharp wave 1) precedes a jerk in cortical myoclonus by 20–40 ms depending on whether the muscle under investigation is in the upper or lower limb,34 2) follows a jerk in brainstem or reticular myoclonus, or is 3) absent that may suggest spinal myoclonus. Most organic jerks including majority of patients with tics do not have a BP prior to the movements.35 However a BP preceding the choreic movements may be observed in patients of choreoacanthocytosis and some patients with tics and those with myoclonus accompanying dystonia may have an abbreviated BP preceding the abnormal movements.25
Stimulus-evoked jerks, such as that seen in stimulus-sensitive myoclonus of cortical origin have giant cortical SSEP, short latency (usually < 100 ms, depending on the muscle analyzed), short duration EMG bursts, and a characteristic descending (craniocaudal) pattern of muscle recruitment.23 In pathological enhancement of startle response or hyperekplexia there is stereotyped non-habituating early response in sternocleidomastoid to sound or tap to the mantle region.23
In cortical reflex myoclonus, LLR is enhanced, with a C reflex recorded from the thenar muscle at a latency of around 45 ms after stimulation of the median nerve at the wrist32 (Figure 5B). In some patients, the enhanced C reflexes can be recorded also from more proximal muscles of the stimulated upper extremity with shorter latency and even from the opposite (nonstimulated) hand muscle.30
In reticular reflex myoclonus, the LLR is enhanced without enhancement of cortical SSEP. The reflex myoclonus first involves bulbar muscles such as the sternocleidomastoid and trapezius muscles, and subsequently the more rostral cranial muscles (such as the facial muscles) and caudal muscles (such as limb muscles) are involved.36
Psychogenic jerks
Electrophysiological evaluation is helpful to differentiate psychogenic from organic jerks25 In psychogenic jerks a well organized triphasic pattern of activation of agonist and antagonist muscles are common and the EMG bursts are of long duration (usually > 70 ms). Stimulus-evoked jerks or jumps with a mean latency in excess of 100 ms suggest voluntary or psychogenic jerks.25 A BP may precede psychogenic jerks.37
The following findings point against a psychogenic jerk: 1) giant cortical somatosensory evoked potentials, 2) short duration EMG bursts, 3) characteristic descending patterns of muscle recruitment (suggesting a cortical myoclonus), and 4) presence of LLR/C reflex.
Clinically it may be difficult to separate psychogenic jerks from tics and myoclonus. Differentiation of psychogenic jerks from organic jerks has been often based on duration of EMG activity of a burst, and the pattern of EMG burst (Table 3). Jerks associated with EMG duration of < 70 ms is likely to be an organic jerk, particularly when there is co-contraction of agonist and antagonist muscle pairs.
A well organized triphasic pattern activation of agonist and antagonist muscle pairs, and a prolonged duration of EMG burst favours psychogenic jerk. Spontaneous jerks preceded by a BP (premovement potential) is likely to be psychogenic, but BP may be recorded in chorea of choreocacanthocytosis,38 and an abbreviated BP can be seen in tics39 and in patients with myoclonus accompanying dystonia.40
Stimulus sensitive psychogenic jerks, unlike organic jerks, have varied latency of onset of jerks (muscle activity) from the causative stimulus (latency) from trial to trial. The latencies are greater than seen in reflex myoclonus of cortical or brainstem origin or longer than the fastest voluntary reaction times of normal subjects.23 The mean latency of > 100 ms suggests voluntary or psychogenic jerks.25 Other features may include variable patterns of muscle recruitment within each jerk and, significant habituation with repeated stimulation.23
In psychogenic jerks mimicking generalized myoclonus, the response may reduce or stop after repeated stimuli, mimicking normal (physiological) startle response. The presence of giant cortical somatosensory-evoked potentials, short EMG bursts, and characteristic descending pattern of muscle recruitment point towards cortical myoclonus.23
Evaluation of psychogenic dystonia
Compared to tremor and jerks, electrophysiological characterization of dystonia is difficult. The protocol of evaluation is similar, consisting of multichannel surface EMG from the symptomatic muscles for prolonged periods during abnormal movements (spasms), rest, and with voluntary contraction. Effort should be made to include the agonists and antagonists to detect the presence of co-contraction, and the unaffected muscles of the same or opposite limbs to document the presence of overflow dystonia. Finally, the patients should be asked to perform various tasks which can induce or modify the ongoing spasms, such as mental arithmetic, finger tapping and opening and closing fist of the unaffected hand, writing (in patients with writer’s cramp), etc.
EMG is analyzed to determine: 1) the pattern of EMG activity in agonists and antagonists during spasm 2) duration of each EMG burst 3) the regularity of occurrence of EMG bursts, 4) presence of overflow activity in remote muscles while performing discrete voluntary acts, and, 5) the difference in the degree of EMG activity (area of rectified EMG) between epochs with and without muscle spasms.
Other investigations which are often useful to characterize an organic dystonia or a dystonic syndrome include: 1) H-reflex recovery curve, 2) mechanically and electrically induced long-latency muscle stretch reflex, 3) reciprocal inhibition between antagonistic muscles, 4) brainstem reflexes such as blink reflex, 5) cortical SEP 6) BP and contingent negative variation (CNV), and 7) transcranial magnetic stimulation.
The following electrophysiological findings are often present, in varying combinations, in organic dystonia. Therefore, in a given patient suspected of psychogenic dystonia, presence of any of the following should warrant a revision in diagnosis and search for underlying cause of dystonia.
Abnormalities of EMG and kinematic studies
The abnormalities include 1) Co-contraction (often up to several seconds) of agonist and antagonist muscle pairs during dystonia. However, this feature is true for voluntary (psychogenic) spasm and may not be of value, 2) repeated short bursts of EMG activity superimposed on the prolonged spasms, which may result in superimposed action and postural tremors,41,42 slow myorhythmia43 or myoclonic jerks,44 depending on the duration and regularity of these jerks, 3) replacement of the normal di-or tri-phasic pattern of activation of agonists and antagonists muscles during voluntary movements by prolonged bursts with a resultant overlap of agonist and antagonist activities, 4) the voluntary movements of the distal part of the limbs may be accompanied by inappropriate activity of remote proximal muscles,45 and 5) an abnormal synchronizing drive at certain frequencies in dystonic muscles.
Abnormalities of spinal cord reflexes
These include abnormalities of H-reflex recovery curve,46 LLR,47 and breakdown of normal pattern of reciprocal inhibition between opposing muscles.48
Abnormalities of brainstem reflexes
These include abnormalities of blink reflex and its recovery cycle in cranial dystonia49,50 as well as in cervical and generalized dystonia even without blepharospasm.51
Abnormalities of SSEPs
The findings are controversial. Late components of SSEP to median nerve stimulation (N30) has been reported to be enlarged in writer’s cramp,52 but normal53 or reduced in size in spasmodic torticollis without hand dystonia.54
Abnormalities of premovement potentials
In primary as well as secondary dystonias abnormalities (mainly reduced amplitudes) of the initial slow and/or the later steep components of BP.55,56 The amplitude of the late component of CNV is reduced in torticollis when patients are asked to rotate their head to either side depending on a signal, and in patients with writer’s cramp while performing hand movements.57
Abnormalities of transcranial magnetic stimulation
A number of abnormalities have been described in dystonia, which include abnormal recruitment pattern, suggesting greater excitability of motor cortex in patients with primary dystonia, reorganization of cortical excitability in patients with writer’s cramp, reduced short interval intracortical inhibition in patients with focal, task-specific primary dystonia when tested at rest, and reduced EMG silent period and changes in long interval intracortical inhibition in patients with dystonia.58–60
Several electrophysiological abnormalities have been reported in dystonia, but the specificity and sensitivity of these findings are not enough to differentiate between organic and psychogenic dystonia.51 In cervical dystonia EMG recording showed a pathological common drive manifest as significant coherence at 4–7 Hz band between sternocleidomastoid and splenius capitis.61
Abnormalities in dystonic torticollis include abnormal low-frequency common drive. A similar abnormality has been reported in the lower limbs of 10 out of 12 symptomatic patients with DYT1 dystonia, but not psychogenic dystonia or normal controls.62 Other abnormality in organic dystonia include impaired reciprocal inhibition of H-reflexes.63
Psychogenic parkinsonism
Electrophysiological evaluation of psychogenic parkinsonism is difficult and there is paucity of reports in the literature. Electrophysiology can help in differentiating a true rest tremor from a psychogenic tremor by methods described previously. Parkinson’s disease is characterized by altered cortical excitability by TMS studies, which if present may support a diagnosis of organic parkinsonism.
Zeuner et al.64 measured postural wrist tremor with accelerometry in 6 patients with psychogenic tremor, 11 with essential tremor and 12 with parkinsonian tremor. Tremor was measured in one hand, while the other hand either rested or tapped to an auditory stimulus at 3 and 4 or 5 Hz. The patients with psychogenic tremors showed larger tremor frequency changes and higher intraindividual variability while tapping. The authors concluded that accelerometry could be an useful tool to differentiate psychogenic from essential and parkinsonian tremor.
Benaderette et al.65 evaluated the concordance between independent clinical, electrophysiological, and [123I]-FP-CIT SPECT scan explorations as a staged procedure for an accurate diagnosis in 9 patients referred with a diagnosis of suspected psychogenic parkinsonism. Three patients were reclassified as pure psychogenic parkinsonism, 6 with a form of combined psychogenic parkinsonism and Parkinson’s disease, and none with pure Parkinson’s disease (PD). Electrophysiological recordings showed the characteristics of psychogenic tremor in 5 of 7 patients with tremor. In two of these 5, PD tremor was also recorded. SPECT scan results were abnormal in five of 9 patients. The authors concluded that electrophysiology contributes to the clinical diagnosis of psychogenic tremor and may help confirm associated organic PD tremor.
Limitations of electrophysiological characterization of PMD
While there is unequivocal role of electrophysiology in supporting a diagnosis of PMD, there are several limitations. Some patients with bilateral psychogenic tremor may be able to maintain independent oscillation frequencies on each side,66 which suggest that there may be another non-voluntary mechanism of tremulousness operating in some patients with psychogenic tremor. Williams et al.67 reported a patient with probable psychogenic propriospinal myoclonus where electrophysiological findings were consistent with organic propriospinal myoclonus (short EMG burst duration and slow spinal conduction). It has also been shown that healthy volunteers simulating propriospinal myoclonus have similar electrophysiological recordings except for long EMG burst durations,68 implying that that electrophysiological parameters alone may not be sufficient to identify “organic” propriospinal myoclonus.67 More recently, van der Salm et al.69 reported that 34 of the 35 cases referred with a diagnosis of propriospinal myoclonus had psychogenic myoclonus. However, the authors did find many cases with electrophysiological characteristics “classically” described in organic propriospinal myoclonus. Finally though a BP is expected in a psychogenic jerk and not an organic jerk, it may be recorded in chorea of choreocacanthocytosis, and an abbreviated BP can be seen in patients with tics, and myoclonus accompanying dystonia.
In summary, electrophysiologic techniques have a definite role in the diagnosis of psychogenic tremor and myoclonus, and may provide sufficient clues to rule out an organic dystonia. However caution should be exerted in the interpretation of the results to avoid both false positive and false negative diagnosis of PMD.
Notes
The author has no financial conflicts of interest.


