Asymmetric Parkinsonism With Progressive Nigrosomal Change Secondary to Kernohan’s Notch Phenomenon
Article information
Dear Editor,
Kernohan’s notch phenomenon is a paradoxical compression of the contralateral midbrain against the tentorium after transtentorial herniation that results in false localization signs of ipsilateral neurological symptoms, particularly hemiparesis [1]. Although midbrain compression may lead us to expect secondary parkinsonism, similar to patients with parkinsonism after solitary nigral lesions [2], there are only three case reports of parkinsonism secondary to Kernohan’s notch phenomenon [3-5]. All three cases exhibited imaging evidence of asymmetric loss of striatal dopaminergic input, but no report presented changes occurring in nigrosome 1, which contains a large proportion of nigrostriatal dopaminergic neurons within the substantia nigra and becomes invisible in magnetic resonance (MR) images in Parkinson’s disease (PD) [6]. In this report, we present a patient with slowly progressive parkinsonism secondary to Kernohan’s notch phenomenon, showing asymmetric striatal dopaminergic loss and longitudinal changes in the nigrosome.
A 40-year-old woman developed acute intracranial hemorrhage due to the rupture of an arteriovenous malformation. An emergency computed tomography (CT) scan exhibited a large hematoma in the right parieto-occipital lobe and a subdural hematoma over the right frontal cortex, pushing the right hemisphere beyond the falx and tentorium (Figure 1A). She underwent emergency decompressive craniotomy and subsequent coil embolization of arteriovenous malformation, and a postoperative CT scan showed removal of most of the hematoma and relief of herniation. After recovery from acute illness, she showed right-side dominant quadriparesis, which gradually improved to normal motor power at 1 month postoperation, leaving left homonymous hemianopsia as permanent sequelae. She was able to walk normally with a mildly reduced right arm swing at 2 months after the operation but began to feel slowness and clumsiness in her right limbs, which gradually worsened until her first visit to our movement disorder clinic at 20 months postoperation. The patient had no past medical history and no symptoms suspicious for rapid-eye-movement sleep behavior disorder (RBD). On neurologic examination, she showed only a very fine postural tremor in her right fingers without dystonia or resting tremor. Severe bradykinesia and mild rigidity were observed in her right limbs, while her left limbs were mildly slow without rigidity. She walked slowly with a moderately short stride and markedly reduced both arms swings, dragging both her feet. She also exhibited plantar flexion dystonia in her right foot during walking, which worsened her right leg dragging. However, motor power and deep tendon reflex were normal in all four limbs.
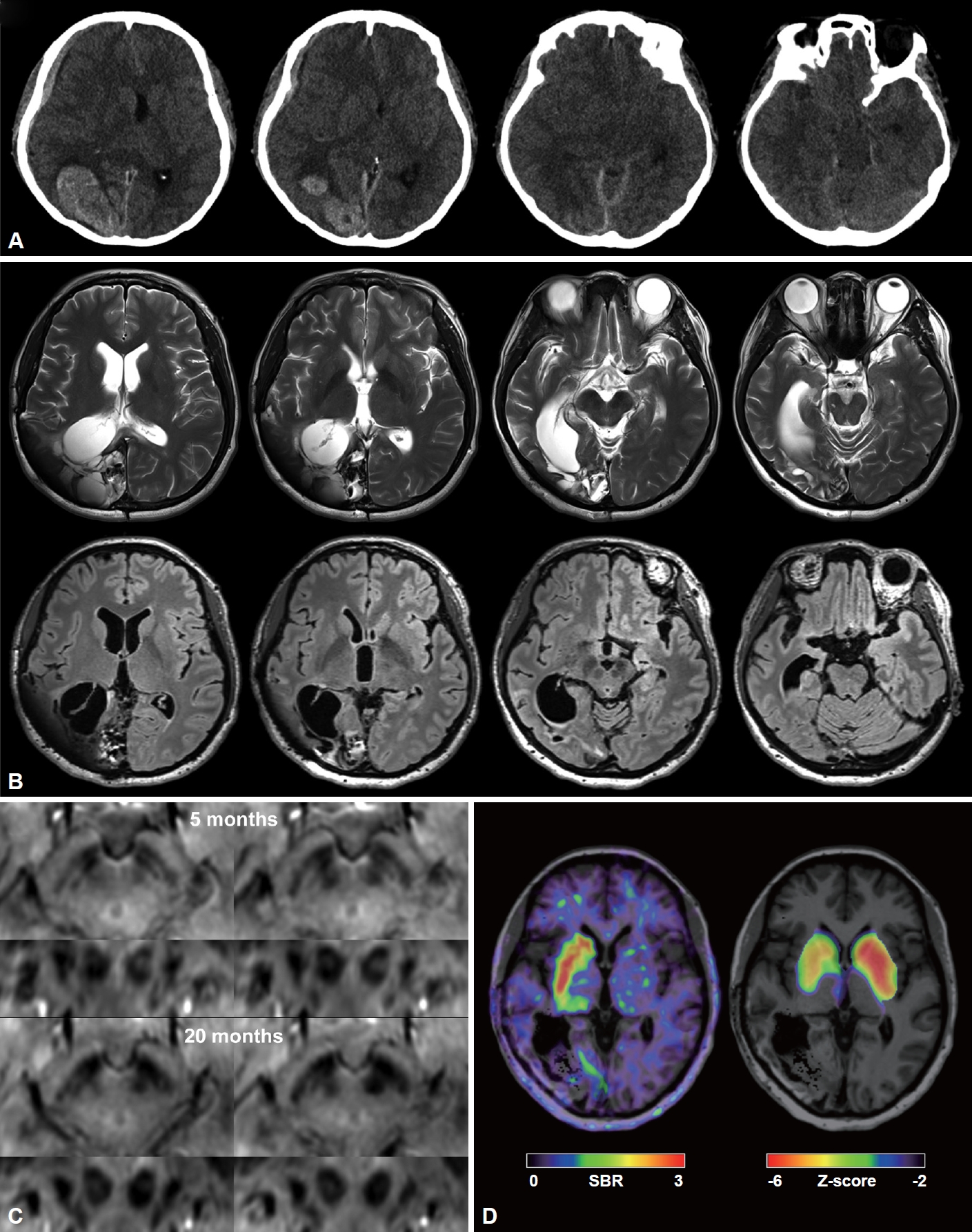
Multimodal imaging studies showing temporal changes in hemorrhage, nigrosomes, and resulting striatal dopaminergic deficits. A: Initial brain CT images show a large hematoma in the right parieto-occipital lobe and a subdural hematoma at the right frontal convexity compressing subcortical structures. B: Brain MR images at 20 months after craniotomy show cystic encephalomalacia in the right parietooccipital lobe in T2-weighted image (upper row) and FLAIR images (lower row) without any visible abnormality in the basal ganglia, thalamus, or midbrain. C: In the susceptibility-weighted angiography images, nigrosome 1 is clearly visible on both sides at 5 months after craniotomy. However, left nigrosome 1 is invisible, and right nigrosome 1 is weakly visible at 20 months after craniotomy. D: 18F-FP-CIT PET at 20 months after craniotomy shows markedly reduced uptake in the entire left striatum and moderately reduced uptake, particularly in the right anterior striatum. The Z score map was created by comparison with the in-house 18F-FP-CIT PET normative data of 71 healthy controls. SBR, specific binding ratio.
T2-weighted and fluid-attenuated inversion recovery (FLAIR) brain MR images at 20 months postoperation showed huge cystic encephalomalacia in the right parieto-occipital lobe without residual hematoma (Figure 1B). Interestingly, nigrosome 1 was clearly visible on both sides of the substantia nigra in the susceptibility-weighted angiography images at 5 months postoperation, but they became only weakly visible on the right side and completely invisible on the left side at 20 months postoperation (Figure 1C). An N-(3-fluoropropyl)-2β-carboxymethoxy-3β-(4-iodophenyl) nortropane (18F-FP-CIT) positron emission tomography (PET) scan at 20 months postoperation exhibited marked loss of uptake in the entire left striatum (5% and 9% of mean caudate and putaminal specific binding ratio (SBR) of healthy controls, respectively) and moderately reduced uptake, particularly in the right anterior putamen and caudate (22% and 27% of mean caudate and putaminal SBR of healthy controls, respectively) (Figure 1D).
After a trial of single levodopa challenge with 100/25 mg of levodopa/carbidopa, her Movement Disorder Society-sponsored Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) motor score improved from 28 to 24, her gait speed was 21%, and her right hand timed motor tests were 20%–30%. We started antiparkinsonian medication with pramipexole extended release 0.375 mg q.d. and later increased to 1.5 mg q.d. with amantadine 100 mg q.d., which mildly improved her gait and bradykinesia in the right limbs and eliminated plantar flexion dystonia in the right foot during the 9-month follow-up period.
Direct compression by a space-occupying lesion around the midbrain as well as lesions involving the midbrain can cause secondary parkinsonism [2]. However, due to the serious underlying conditions causing Kernohan’s notch phenomenon and residual motor weakness hiding parkinsonian symptoms even in survivors, secondary parkinsonism after Kernohan’s notch phenomenon is extremely rare [1]. Compressive damage to the nigrostriatal fibers and petechial hemorrhage or cytotoxic edema involving the substantia nigra were suggested to be the mechanisms explaining secondary parkinsonism [1].
All three previously reported patients with secondary parkinsonism after Kernohan’s notch phenomenon exhibited delayed onset (1 to 18 months) of ipsilateral parkinsonism; nevertheless, the striatal dopaminergic input was markedly reduced in the contralateral striatum and mildly reduced in the ipsilateral striatum [3-5]. Unfortunately, no information about the integrity of motor function in the contralateral limbs was available in their reports. Unlike these patients, our patient exhibited bradykinesia and loss of striatal dopaminergic input on both sides with marked asymmetry. Bradykinesia in her left limbs may be attributable to the effect of compression of the right substantia nigra by herniation or even direct compression of the right striatum by hematoma. We suspect that the patient may have had coincidental degenerative parkinsonism. However, an absence of RBD symptoms and a striatal 18F-FP-CIT uptake pattern atypical of classic PD [7] raise the possibility that the loss of striatal 18F-FP-CIT uptake was caused by direct compression of the midbrain rather than degenerative parkinsonism.
It is very interesting to note that the patient exhibited dramatic longitudinal changes in nigrosome 1. Nigrosome 1, clearly visible on both sides at 5 months postoperation, completely disappeared on the left side and weakened on the right side. To the best of our knowledge, our patient is the first to show the disappearance of nigrosome 1 after a compressive lesion on the midbrain. Moreover, this is the first report demonstrating longitudinal changes in nigrosome 1. Considering that the patient already showed parkinsonian symptoms at 5 months postoperation, we suspect that the disappearance of nigrosome 1 is delayed after damage to nigrostriatal neurons, and that sufficient loss of nigrostriatal neurons is required for the disappearance of nigrosome 1.
Notes
Ethics Statement
This study was approved by the Institutional Review Board of Gangnam Severance Hospital with a waiver of the requirement for informed consent (approval no. 3-2021-0067).
Conflicts of Interest
The authors have no financial conflicts of interest.
Funding Statement
This research was supported by a faculty research grant from Yonsei University College of Medicine (6-2021-0094).
Author Contributions
Conceptualization: Chul Hyoung Lyoo. Data curation: Han-Kyeol Kim, Sung Jun Ahn, Chul Hyoung Lyoo. Formal analysis: Han-Kyeol Kim, Sung Jun Ahn, Chul Hyoung Lyoo. Funding acquisition: Chul Hyoung Lyoo. Investigation: Han-Kyeol Kim. Methodology: Han-Kyeol Kim. Project administration: Han-Kyeol Kim. Resources: Han-Kyeol Kim, Sung Jun Ahn. Software: Chul Hyoung Lyoo. Supervision: Min Seok Baek, Sung Jun Ahn, Chul Hyoung Lyoo. Validation: Min Seok Baek, Sung Jun Ahn. Visualization: Han-Kyeol Kim, Chul Hyoung Lyoo. Writing—original draft: Han-Kyeol Kim. Writing—review & editing: Han-Kyeol Kim, Chul Hyoung Lyoo.
