Mosapride-Induced Movement Disorders
Article information
Dear Editor,
Drug-induced movement disorders are mainly caused by dopaminergic receptor blocking agents in the striatonigral pathway [1]. Common offending drugs causing movement disorders are antipsychotic drugs that directly block nigral dopaminergic receptors. Along with dopamine antagonists, gastrointestinal (GI) prokinetics have been known to cause various drug-induced movement disorders. Serotonin 5-HT4 receptor agonists have been widely used for improving symptoms of various G-I motility disorders [2]. Metoclopramide, levosulpiride, cisapride, mosapride, and renzapride are included in this group. Among these drugs, metoclopramide and levosulpiride also act on peripheral and central dopamine D2 receptors. Therefore, parkinsonism and movement disorders associated with these drugs are well known [3]. Mosapride is a gastroprokinetic agent that acts as a selective 5-HT4 agonist [2]. The primary active metabolite of mosapride accelerates gastric emptying throughout the whole GI tract in humans. Unlike metoclopramide and levosulpiride, mosapride is known as a G-I-prokinetic that does not cause parkinsonism or other drug-induced movement disorders because it does not act directly on dopamine receptors. Since cisapride was withdrawn from the market due to the risk of cardiac arrhythmia with Q-T prolongation, clinical trials of 5-HT4 receptor agonists have mainly focused on cardiac side effects [4]. Thus, druginduced parkinsonism (DIP) and other drug-induced movement disorders have been relatively ignored in clinical trials. We describe two patients with parkinsonism and one patient with tardive dyskinesia (TD) who developed neurological symptoms after taking mosapride. Case 1: A 79-year-old female patient with a history of hypertension, heart failure, asthma, and trigeminal neuralgia was hospitalized due to a rapid, progressive gait disturbance for 3 months. She had taken medications, including amlodipine, valsartan, nicorandil, trimetazidine, pranlukast, levocetirizine, carbamazepine, amitriptyline, and alprazolam. She had two episodes of DIP due to levosulpiride and flunarizine over the past 10 years. In both episodes, she recovered from DIP after the cessation of the offending drugs. Four months before hospitalization, she fell accidentally and suffered a leg injury without fracture. The patient took medications, including mosapride, for pain control and physical therapy at the orthopedic clinic. Although her leg injury improved, she developed gait disturbance over 3 months. She experienced shuffling gait and frequent falling forward. Initial neurological examination showed symmetric bradykinesia that was more severe in the lower extremities. She was unable to walk independently due to severe freezing of gait and had a tendency to fall forward. Her Unified Parkinson’s Disease Rating ScalePart III (UPDRS-III) score was 14. Initial brain magnetic resonance imaging (MRI) showed no structural lesions responsible for her parkinsonism (Figure 1A). 18F-Fluorinated N-3-fluoropropyl-2-beta-carboxymethoxy-3-beta-(4-iodophenyl) nortropane (18F-FP-CIT) positron emission tomography (PET) scans showed normal uptake of the dopamine transporter (DAT) in the striatum (Figure 1B). Laboratory findings, including complete blood count (CBC), routine chemistry tests, electrolyte levels, thyroid function tests, and vitamin B12 levels, were normal. After the cessation of mosapride but the continuation of the other drugs, her gait difficulty improved. At discharge, bradykinesia remained; however, she had almost completely recovered after several months. The UPDRS-III score at 6 months was 3 (posture: 2, body bradykinesia: 1). Parkinsonism has not recurred in over 2 years. Case 2: An 82-year-old male patient, who had used a cane and pain control medication for spinal stenosis for several years but had no difficulty walking, was admitted to our hospital due to a rapid, progressive gait disturbance for 2 months. He had no medical history. He reported initial shuffling and festination when walking and eventually needed a wheelchair by the time he visited the hospital. On neurological examination, he could not walk and showed severe bradykinesia and rigidity in both arms and legs. After admission, we confirmed that mosapride was included in the drugs prescribed for spinal stenosis for 6 months. The initial UPDRS-III score was 44. Brain MRI was normal (Figure 1A). Although global cerebral atrophy was seen, his Mini-Mental State Examination score was 25/30, and he did not have cognitive impairment in daily life. In addition, he did not show vertical gaze limitation. 18F-FP-CIT PET scans showed normal uptake of the DAT in the striatum (Figure 1B). Laboratory findings, including CBC, routine chemistry tests, electrolyte levels, thyroid function tests, and vitamin B12 levels, were normal. He started empirically taking 300 mg of levodopa per day along with cessation of mosapride because his parkinsonism was severe. His gait difficulty and parkinsonism rapidly improved. After receiving rehabilitation for 1 month, he was discharged from the hospital while walking with a cane. Levodopa was tapered out for 6 months. The last UPDRS-III score was 6 (arising from chair: 2, gait: 2, and posture: 2), and overt parkinsonism has not recurred. Case 3: An 80-year-old female patient visited a neurology outpatient clinic due to a rapidly progressing gait disturbance and frequent falling for 3 months. She had been regularly examined twice a year due to a mild cognitive impairment and had taken choline alfoscerate prescribed bythe neurology department for 4 years. At the last visit, before developing her falling episodes, she did not have gait difficulty or parkinsonism. She had hypertension, hyperlipidemia, and carotid artery stenosis for 7 years and had taken nifedipine, hydrochlorothiazide, and atorvastatin. On neurological examination, she showed involuntary movements in four extremities, along with orolingual dyskinesia. She did not show any abnormal posture of the neck, trunk, or extremities. The involuntary movements in the limbs were irregular and random. The amplitude of the involuntary movements was higher in the legs than in the arms. She showed an unsteady gait due to dyskinesia in her legs. However, her motor power was intact. Brain MRI showed no structural lesions (Figure 1A). Laboratory findings, including CBC, routine chemistry tests, electrolyte levels, and thyroid function tests, were normal. She suffered from GI problems and had taken medications, including mosapride, for approximately 6 months. After stopping this drug, dyskinesia of the whole body and gait difficulty improved over the next 4 months, confirming TD.
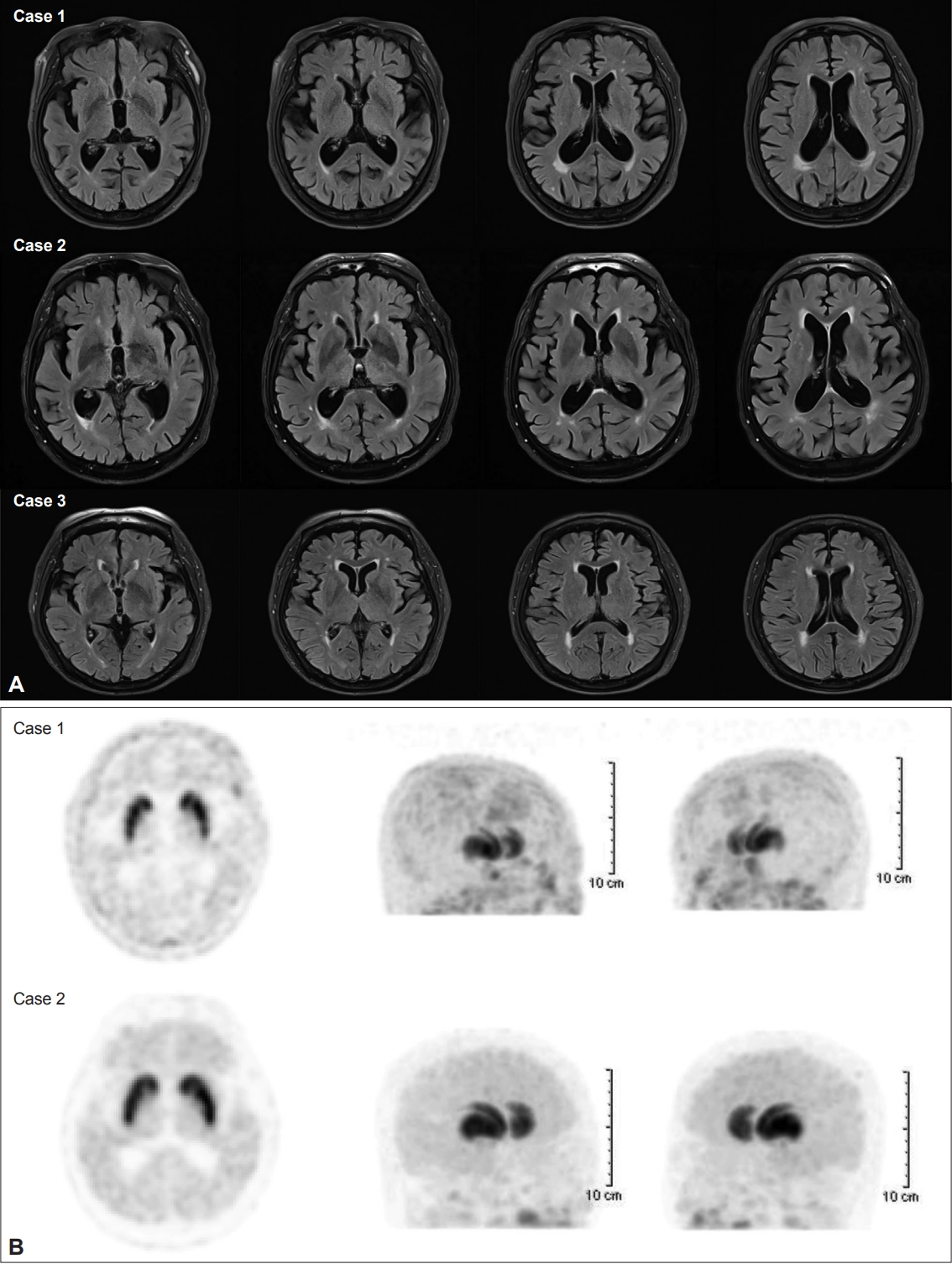
A: Brain magnetic resonance imaging scans of the three patients showed no significant lesions responsible for parkinsonism. B: 18F-Fluorinated N-3-fluoropropyl-2-beta-carboxymethoxy-3-beta-(4-iodophenyl) nortropane positron emission tomography in Patient 1 and Patient 2 showed normal uptake of dopamine transporters in the striatum.
We diagnosed these three patients as having mosapride-induced parkinsonism and TD based on the fact that they had no parkinsonian symptoms or dyskinesia before taking the drug and developed neurological symptoms after taking this drug. The parkinsonian symptoms disappeared after stopping mosapride. Brain MRI in all patients showed no apparent structural lesions responsible for parkinsonism, including normal pressure hydrocephalus and significant ischemic lesions. Moreover, DAT scans performed in Cases 1 and 2 showed normal DAT uptake in the striatum. These findings in Cases 1 and 2 are consistent with a diagnosis of DIP. In Case 3, the duration of mosapride ingestion and symptoms of dyskinesia were 6 and 3 months, respectively. In addition, the dyskinesia improved over 4 months after mosapride cessation. According to the definition of TD, she was diagnosed with TD based on the temporal clinical course [5]. In addition, the definition of TD has been expanded to any combination of hyperkinetic movement disorders in any part of the body [5]. Secondary chorea other than TD was excluded using brain MRI and laboratory tests; however, tests for immunological disorders associated with chorea could not be performed.
Mosapride selectively acts on the 5-HT4 receptor without blocking peripheral and central dopamine receptors [2]. Therefore, it is perceived to be safe with regard to DIP and other movement disorders. Indeed, mosapride-induced parkinsonism or movement disorders have not been reported since this drug was launched. However, the Korea Pharmaceutical Information Center reported one patient who experienced mosapride-related lactation in a post-market surveillance. This finding supports the idea that mosapride can be transported into the central nervous system (CNS) and increase prolactin secretion via the D2 receptor.
Various G-I prokinetics and atypical antipsychotics have been developed as being free from striatal dopaminergic antagonism. For example, levosulpiride was widely used in the Korean pharmaceutical market as a G-I prokinetic that did not affect the CNS until it was revealed that levosulpiride is among the common offending drugs causing DIP and movement disorders [6]. Atypical antipsychotics have also been developed as being free from extrapyramidal side effects because typical antipsychotics, including haloperidol and perphenazine, induce many kinds of extrapyramidal syndromes in almost all patients [3]. Although atypical antipsychotics have lessened the prevalence of DIP and movement disorders, they also carry a risk of causing extrapyramidal symptoms (EPS), particularly for elderly individuals. Since mosapride is a relatively recent drug, the possibility of unknown adverse reactions should be acknowledged.
It is not clear by what mechanism mosapride-induced parkinsonism and TD developed in our patients. As noted, there is a possibility that mosapride is transported into the CNS and weakly blocks nigrostriatal dopaminergic receptors. Because all three patients presented here were very old, they could have been more vulnerable to developing EPS than participants in the clinical trials of mosapride; therefore, they could be more likely to develop mosapride-induced parkinsonism and TD. A recent clinical study reported that Motilitone, a 5-HT4 agonist with D2 receptor antagonist actions, improved G-I dysfunction without worsening parkinsonism in patients with Parkinson’s disease (PD) [7]. Since almost all PD patients take levodopa or other dopaminergic medications, even though parkinsonism is worsened by G-I prokinetics, it may be masked or regarded as disease progression; thus, the adverse effects of the G-I prokinetic may be ignored.
The three patients described here provide evidence of the possibility of dopaminergic receptor antagonism by mosapride in the nigrostriatal system. Clinicians need to be cautious when prescribing this drug, particularly for elderly individuals.
Notes
Ethics Statement
The requirement of informed consent was waived by the institutional review board.
Conflicts of Interest
The authors have no financial conflicts of interest.
Funding Statement
None
Author Contributions
Conceptualization: Hae-Won Shin. Data curation: Sang-Wook Hong. Formal analysis: Sang-Wook Hong. Investigation: Sang-Wook Hong. Methodology: Hae-Won Shin. Project administration: Hae-Won Shin. Resources: Sang-Wook Hong. Software: Sang-Wook Hong. Supervision: Hae-Won Shin. Validation: Hae-Won Shin. Visualization: Sang-Wook Hong. Writing—original draft: Sang-Wook Hong. Writing—review & editing: Hae-Won Shin.
