Long-Term Outcomes of Deep Brain Stimulation in Pantothenate Kinase-Associated Neurodegeneration-Related Dystonia
Article information
Abstract
Objective
To investigate the long-term clinical outcomes of pallidal deep brain stimulation (GPi-DBS) in patients with pantothenate kinase-associated neurodegeneration (PKAN).
Methods
We reviewed the records of patients with genetically confirmed PKAN who received bilateral GPi-DBS for refractory dystonia and were clinically followed up for at least 2 years postoperatively at two centers in Korea. Pre- and postoperative Burke–Fahn–Marsden Dystonia Rating Scale motor subscale (BFMDRS-M) scores, disability subscale (BFMDRS-D) scores, and qualitative clinical information were prospectively collected. Descriptive analysis was performed for BFMDRS-M scores, BFMDRS-D scores, and the orofacial, axial, and limb subscores of the BFMDRS-M at 6–12, 24–36, and 60–72 months postoperatively.
Results
Five classic-type, four atypical-type, and one unknown-type PKAN cases were identified. The mean preoperative BFMDRS-M score was 92.1 for the classic type and 38.5 for the atypical or unknown type, with a mean BFMDRS follow-up of 50.7 months and a clinical follow-up of 69.0 months. The mean improvements in BFMDRS-M score were 11.3%, 41.3%, and 30.5% at 6–12, 24–36, and 60–72 months, respectively. In four patients with full regular evaluations until 60–72 months, improvements in the orofacial, axial, and limb subscores persisted, but the disability scores worsened from 24–36 months post-operation compared to the baseline, mainly owing to the aggravation of eating and feeding disabilities.
Conclusion
The benefits of GPi-DBS on dystonia may persist for more than 5 years in PKAN. The effects on patients’ subjective disability may have a shorter duration despite improvements in dystonia owing to the complex manifestations of PKAN.
Pantothenate kinase-associated neurodegeneration (PKAN) is a rare autosomal recessive disorder caused by mutations in the PANK2 gene, which encodes the pantothenate kinase 2 enzyme in the coenzyme A synthesis pathway [1-3]. PKAN is associated with heterogeneous neurological manifestations. Movement disorders include dystonia, choreoathetosis, and rigidity, along with pyramidal symptoms and cognitive impairment [2]. Two distinct types of PKAN can be identified based on their clinical manifestations, with classic PKAN typified by earlier onset and rapid progression of symptoms and atypical PKAN with a later onset and a more gradual progression [2-5].
Currently, no disease-modifying therapy exists for PKAN. Symptomatic management options include medications such as baclofen, benzodiazepines, and anticholinergics [5], as well as therapeutic interventions such as intrathecal baclofen and ablative brain operations [6-8]. Since the benefits of deep brain stimulation (DBS) targeting the globus pallidus (GPi) on primary generalized or segmental dystonia have been demonstrated in two prospective trials [9,10], the efficacy of GPi-DBS for dystonia in patients with PKAN has been proposed in a number of cases. A recent meta-analysis of 99 PKAN cases reported improvement in dystonia severity 1 year after DBS implantation, including 87 cases with GPi-DBS [11]. However, data on the long-term prognosis after DBS in patients with PKAN-associated dystonia remain scarce. Herein, we report the long-term clinical follow-up data of patients with PKAN who underwent DBS implantation in two tertiary movement disorder centers.
MATERIALS & METHODS
We reviewed the records of patients who received DBS implantation at Seoul National University Hospital (SNUH) and Asan Medical Center. The inclusion criteria were as follows: patients with 1) genetically confirmed PKAN with a clinical diagnosis of neurodegeneration with brain iron accumulation (NBIA), 2) who received bilateral GPi-DBS for moderate to severe medically intractable dystonia, 3) had been evaluated for Burke–Fahn–Marsden Dystonia Rating Scale motor (BFMDRS-M) score [12] by a movement disorder specialist before surgery was available, and 4) had information available regarding clinical status after DBS implantation for ≥ 2 years postoperatively.
Demographic and baseline clinical data included sex, age at onset, age at diagnosis, age at surgery, the results of genetic testing, family history, initial neurological symptoms and brief clinical history, location of dystonia, and presence of cognitive impairment assessed by Mini–Mental State Examination or detailed pediatric developmental evaluation. Information on preoperative brain magnetic resonance imaging (MRI) findings and dystonia medications was also collected. In agreement with the previous meta-analysis by De Vloo et al. [11], the type of PKAN was determined based on the age at onset, with onset before age 10 classified as classic type, during age 10–14 years as unknown type, and at age ≥ 15 as atypical type.
The severity of dystonia was assessed using the BFMDRS-M both preoperatively and postoperatively under concurrent symptomatic pharmacotherapy for dystonia. BFMDRS-M and BFMDRS disability (BFMDRS-D) scores, if available, were rated postoperatively at each follow-up outpatient visit along with descriptive data on major clinical deteriorations and adverse events. The postoperative BFMDRS score was converted into a percentage of the preoperative score for each patient. An improvement of > 20% in the BFMDRS-M score was considered clinically relevant [2,13].
Descriptive analysis was performed for BFMDRS scores at three time points: 1) 6–12 months, 2) 24–36 months, and 3) 60–72 months postoperatively. To assess the effect of DBS on different anatomical locations, the orofacial (eyes, mouth, speech, and swallowing), axial (neck and trunk), and limb (bilateral arms and legs) subscores of the BFMDRS-M were also reviewed. If the same patient was evaluated more than once within each time point, the score evaluated later was included. Because not all patients were assessed with the BFMDRS throughout the entire follow-up period and some clinical features of PKAN could not be captured by the BFMDRS, descriptive information on adverse events and major clinical exacerbations were also reviewed.
This study was approved by the Institutional Review Boards (IRBs) of the Seoul National University Hospital and Asan Medical Center (IRB number: 2009-106-1157). Informed consent was waived due to the retrospective nature of the study protocol (i.e., database review), although the raw data were collected in a prospective manner. All procedures performed in this study involving human participants were in accordance with the 1975 Helsinki Declaration and its later amendments or comparable ethical standards.
RESULTS
Demographics and clinical characteristics
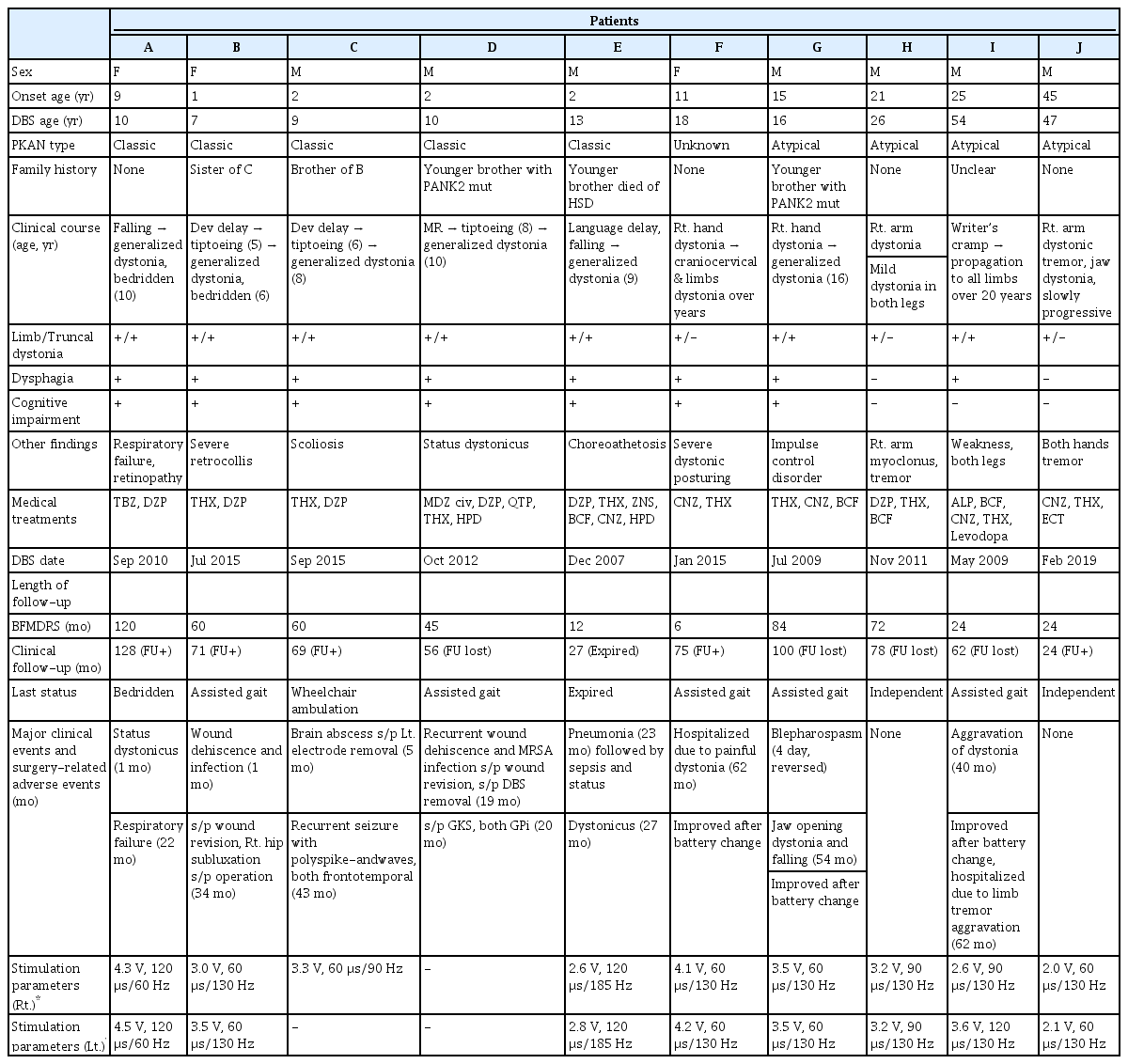
A total of 10 patients met our inclusion criteria and were included in the final analysis (Supplementary Figure 1 in the online-only Data Supplement). The demographic and baseline clinical characteristics of the patients are summarized in Table 1.
The mean age at clinical onset was 13.3 years (range 1–45). Five patients with classic type, four with atypical type, and one with unknown type of PKAN were identified. The average BFMDRS-M score before surgery was 92.1 (range 76.5–108.5) for the classic type and 38.5 (range 15–55) for the atypical and unknown types. By the time of the first DBS implantation, eight patients developed generalized dystonia, as reviewed according to the 2013 dystonia classification criteria [14]. All patients had the typical “eye of the tiger” sign on brain MRI.
Patients were clinically followed up for a mean period of 69.0 months (range 24–128) after the first GPi-DBS implantation. The average BFMDRS-M follow-up period after GPi-DBS was 50.7 months (range 6–120). Dystonia severity information, as assessed by the BFMDRS-M, in the 6–12-month postoperative period was available for 9 patients, at 24–36 months for 7 patients, and at 60–72 months for 5 patients. The BFMDRS-M and -D scores at all three time points were available for 4 patients (patients A, B, C, and G).
Changes in dystonia severity
The mean BFMDRS-M scores improved from 69.0 preoperatively to 61.8 in the postoperative periods of 6–12 months, 63.1 to 39.3 at 24–36 months, and 76.2 to 52.5 at 60–72 months, as shown in Figure 1A. The mean relative changes compared to the individual preoperative scores were -11.3% (range -59.0% to 49.0%), -41.3% (range -66.0% to -7.4%), and -30.5% (range -65.0% to -7.4%), respectively. The percentage of patients who showed > 20% improvement in BFMDRS-M score was 33.3% at 6–12 months, 71.4% at 24–36 months, and 60% at 60–72 months.
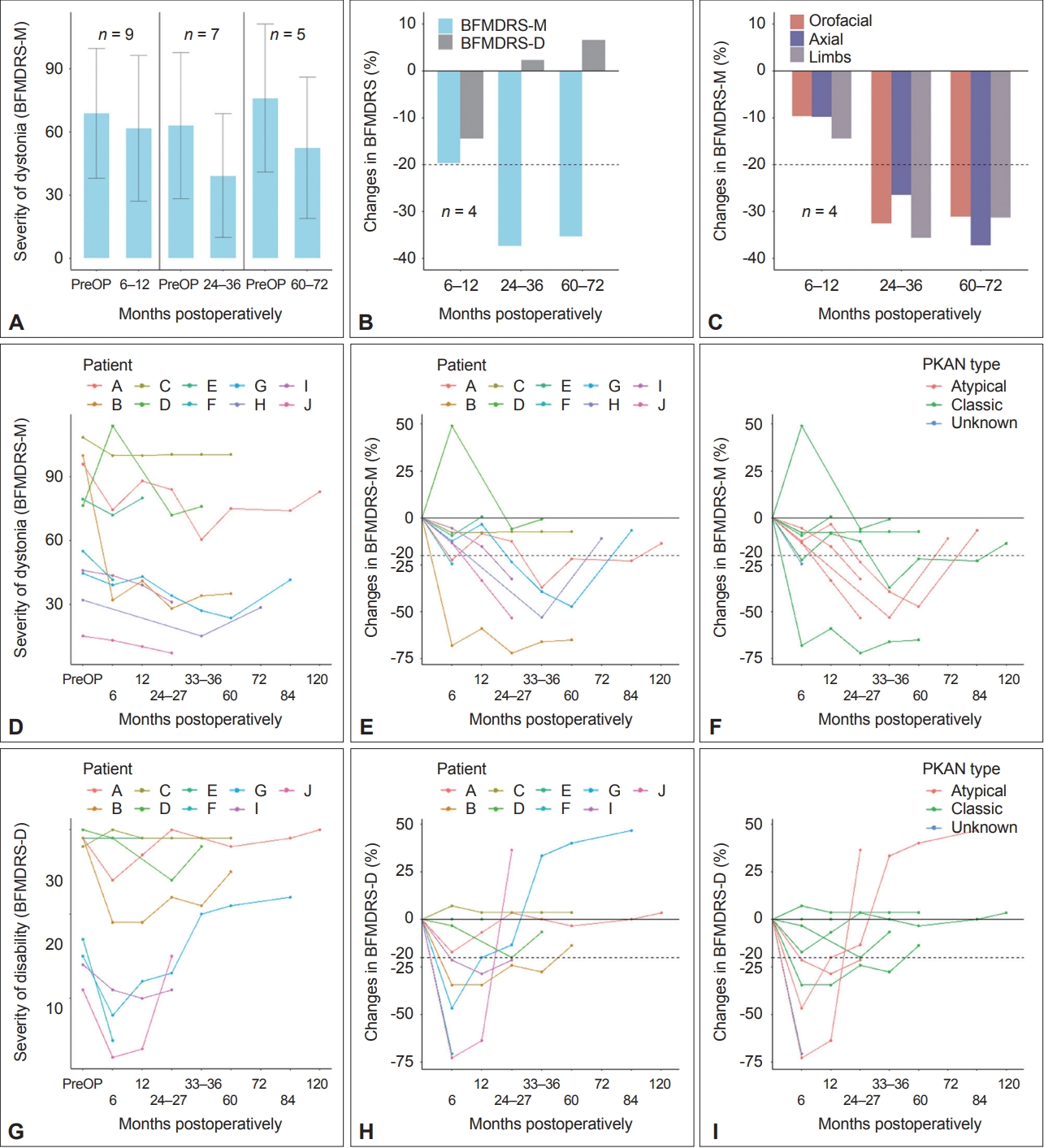
Outcomes of globus pallidus internus deep brain stimulation in patients with pantothenate kinase-associated neurodegeneration (PKAN) assessed by the Burke–Fahn–Marsden Dystonia Rating Scale (BFMDRS). (A) Absolute changes in BFMDRS-M scores at 6–12, 24–36, and 60–72 months postoperatively compared to baseline. Means ± standard deviation (SD) are shown in the (A) bar chart. (B) Serial relative changes in BFMDRS-M scores, BFMDRS-D scores, and (C) subscores of BFMDRS-M compared to baseline in the 4 patients evaluated at all three time points. (D-I) Individual evolutions in absolute (D, G) and relative (E, H) BFMDRS-M and BFMDRS-D scores and colored per PKAN type (F, I). -M, movement subscale; -D, disability subscale; PreOP, preoperative.
In the four patients who underwent full regular evaluations, the mean improvement in BFMDRS-M score was -19.6% (range -59.0% to -3.4%) at 6–12 months, -37.4% (range -66.0% to -7.4%) at 24–36 months, and -35.4% (range -65.0% to -7.4%) at 60–72 months (Figure 1B and E). Serial changes in the orofacial, axial, and limb dystonia scores in these four patients are shown in Figure 1C. At the 60–72-month time point, 3 out of the 4 patients showed > 20% improvement in axial and limb dystonia scores and two in orofacial scores. However, the mean relative changes in disability severity assessed by the BFMDRS-D were -14.5% (range -34.5 to 3.6), +2.33% (range -27.6 to 33.3), and +6.6% (range -13.8 to 40.0) in serial order, with no patient showing > 20% improvement at 60–72 months, suggesting minimal benefits regarding disability from the 24–36-month period (Figure 1B and H). Among the individual items of the disability scale, eating and feeding worsened the most at 60–72 months, with changes of +33.3% (range 0 to 100.0%) and +25.0% (range 0 to 100.0%), while writing showed the largest improvement of -25.0% (range -75.0% to 0). Regarding the provoking factors, which evaluate the circumstances in which dystonia appears [12], the sum of the product of the provoking factor and the weight of each item in the BFMDRS-M decreased by -8.85%, -17.71%, and -18.75% at the three consecutive time points, suggesting that the persistence of dystonia itself improved.
The line graphs of individual patient outcomes are shown in Figure 1D-I. All five patients with atypical and an unknown type of PKAN achieved > 20% improvement in dystonia severity and disability scores after DBS implantation at time points ranging from 6 to 60 months postoperatively, while three of the five patients with classic PKAN did not reach the same level of improvement at any time after the surgery (Figure 1F and I). All BFMDRS-M and BFMDRS-D scores collected in this study are presented in Supplementary Table 1 (in the online-only Data Supplement).
Adverse events and major clinical deterioration after DBS implantation
Eight of the ten patients underwent major clinical events that required hospitalization and/or surgery ≥ 1 year postoperatively. Surgical wound-related problems occurred in three cases. Patient B had a wound healing problem in the left chest that required debridement and wound revision 1 month postoperatively. Patient D experienced recurrent infection and dehiscence of both chest wounds despite repeated debridement and revision. He underwent DBS implant removal 19 months postoperatively, and gamma knife surgery in both parts of the GPi was performed the following month. Patient C developed a surgical site abscess in the left basal ganglia and thalamus, and the left DBS electrode was removed 5 months postoperatively.
Three patients experienced neurological deterioration that improved after DBS battery changes. Patients F and I, who had been lost to follow-up for > 1 year, presented with painful limb dystonia and aggravation of cervical and limb dystonia at 62 and 40 months after DBS implantation, respectively. Patient G presented with worsening of jaw-opening dystonia and falling at 54 months postoperatively despite regular outpatient visits. During the patient’s battery replacement operation, the connection between the battery and the extension cable was found to be discolored, and there was improvement in symptoms after replacing both the battery and cable.
The presenting symptoms were painful limb dystonia in patient F, jaw-opening dystonia and frequent falling in patient G, and aggravation of cervical and limb dystonia in patient I, which occurred 62, 54, and 40 months after DBS implantation, respectively. Patient I underwent another hospitalization to adjust the medication dosage for aggravated limb tremors 62 months postoperatively.
Patient A, who suffered from status dystonicus 1 month postoperatively, experienced resolution of symptoms with continuous intravenous infusion of midazolam but underwent a tracheostomy due to respiratory failure 22 months after DBS implantation. Patient E developed pneumonia 2 years postoperatively, followed by sepsis, gastrointestinal bleeding, and status dystonicus with a lethal outcome. Patient C developed recurrent generalized tonic-clonic seizures 43 months postoperatively, with electroencephalography revealing polyspike-and-wave discharges in both frontotemporal areas.
Neurological status at last clinical evaluation
The neurological status of the patients at the last clinical follow-up was as follows: Among the classic type patients, one patient died during follow-up (patient E). Patient A, who was bedridden preoperatively due to severe generalized dystonia, was followed up for the longest duration (128 months) and remained bedridden with a milder degree of dystonia. Patient B, also bedridden before DBS, was able to stand with assistance, attend elementary school, write by herself, and eat without dysphagia 71 months postoperatively. Patient C ambulated with a wheelchair, remained seizure-free on perampanel, and was feeding on a gastrostomy tube at the 69-month follow-up. Patient D, who experienced status dystonicus before DBS and aggravation of generalized dystonia after DBS electrode removal, remained stable, with improvement in generalized dystonia until 36 months after gamma knife surgery.
Regarding the patients with atypical or unknown types of PKAN, patient F was able to walk independently until 2 years after DBS implantation, but there was worsening of right leg dystonia afterward, required assistance 75 months postoperatively. Patient G experienced frequent falling, gait disturbance, and jaw-opening dystonia 100 months postoperatively. Patients H and J remained neurologically stable, with mild improvement in right arm dystonia at 78 and 24 months postoperatively, respectively. Patient I showed improvement in dysphagia, dysarthria, limb dystonia, and tremor, but there was aggravation of dysphagia and limb tremors 4 years postoperatively, leading her to be dependent on a cane 62 months after DBS implantation.
DISCUSSION
In our investigation of patients with PKAN who received bilateral GPi-DBS implantation for moderate to severe dystonia, all five patients evaluated 60–72 months postoperatively showed improvement in BFMDRS-M scores compared to preoperative evaluations, with three patients showing > 20% improvement. Five out of seven patients with BFMDRS-M scores available 24–36 months post-surgery showed > 20% improvement compared to baseline. Our observations suggest that the benefits of GPi-DBS on dystonia in genetically confirmed PKAN can persist for more than 5 years postoperatively.
Several previous case series and meta-analyses have described the short-term benefits of pallidal stimulation. Timmermann et al. [13] reported a 25.7% improvement in BFMDRS-M scores 9–15 months postoperatively in 23 NBIA patients, including 14 patients with PANK2 mutations. In a meta-analysis of monogenic dystonias, Artusi et al. [15] reported a 27% improvement in dystonia scoring in the PANK2 mutation group 6–12 months postoperatively. A meta-analysis of 99 patients with PKAN showed a 26% improvement in BFMDRS-M scores 12 months after DBS, but this study lacked genetic confirmation in 56% of the patients and used interpolated scores in cases without BFMDRS scores at 12 months [11]. Fewer observations have been made regarding outcomes after 2 years. A case series of five patients with PKAN reported improvement in dystonia 14–36 months postoperatively [16]. Two cases in a series by Castelnau et al. [17] showed sustained improvement after 32 and 42 months, Adamovicová et al. [18] described improvements of 24% and 51% after 4 years in two siblings, and Krause et al. [19] described a 24% improvement after 5 years in one patient; however, in these three cases, secondary progression was observed after the first year. Compared with these previous observations, the present study provides supportive data for GPi-DBS over an extended follow-up period in a larger number of patients with genetically confirmed PKAN.
In our study, the effect of GPi-DBS was more impressive in patients with atypical or unknown type PKAN compared to the classic type in terms of the proportion of patients who achieved > 20% improvement in dystonia severity at any time after the operation (100% vs. 40%; 100% vs. 50% with the exception of patient D, a classic-type patient who underwent DBS due to status dystonicus). This is consistent with a previous meta-analysis of 1-year DBS outcomes, in which a higher proportion of atypical-type patients improved by 30% in BFMDRS-M scores compared to the classic-type patients (73% vs. 34%) [11]. Higher preoperative dystonia scores and having an atypical type of PKAN were correlated with greater 1-year improvement in the same study [11]. Together, these findings may have some implications for possible candidates for DBS in patients with medically refractory PKAN-associated dystonia. However, as observed in our patients, it is known that dystonia is generally less severe in atypical disease [2], which warrants cautious interpretation of the type-specific efficacy of GPi-DBS and calls for controlled studies in the population with the disease. Regarding the benefits of GPi-DBS on status dystonicus in patients with PKAN, a few case reports described relatively favorable outcomes after surgery with moderate controls of dystonia and no further recurrence of status dystonicus, although the follow-up periods were limited to 1 year or shorter [6,20]. Patient D required DBS implant removal due to recurrent wound-related problems that prevented observation of the natural course after surgery, but at least there was no recurrence of status dystonicus, while the dystonia severity scores did not improve remarkably. Further accumulation of reports may shed light on the extent of surgical benefits on PKAN-related status dystonicus. In addition, whether to perform DBS on patients who are already bedridden, as in our patients A and B, can be an issue. In our cases, both patients showed improvement after surgery; patient B became able to walk with assistance, and patient A had partial improvement in dystonia. However, it is difficult to predict whether and to what extent improvement will occur before surgery. We suggest that in the absence of curative treatment for PKAN, whether DBS is an appropriate decision in these cases should be determined individually by taking into account the disabilities caused by additional neurological symptoms, careful drug optimization, and the patient’s and family’s willingness to undergo surgery after proper counseling.
In both classic and atypical PKAN, the disability scores showed minimal improvement or re-exacerbation compared to baseline after years of pallidal stimulation, as opposed to the sustained improvement in dystonia severity. This was also observed in the analysis of four patients with a full regular evaluation despite the sustained benefits to orofacial, axial, and limb dystonia. A similar finding has been previously reported in a case series of 23 patients with NBIA, including 14 patients with PKAN, although the maximal follow-up period was 9–15 months in the study [13]. It is noteworthy that in our study, the mean disability scores worsened at 60–72 months from 24 to 36 months. Although patients reported continuous improvement in writing, deterioration of eating and feeding contributed the most to this progression. Given that eating and feeding require both motor strength and complex coordination of bulbar and extrabulbar muscles, this discrepancy is thought to reflect the course of PKAN as a complex neurological disorder that progresses despite surgery. Although dystonia is a nearly consistent manifestation of the disease, studies on the natural course of PKAN show that pyramidal (25%), cognitive (29%), and extrapyramidal (98%) features are common in classic PKAN, resulting in most patients becoming nonambulatory within 15 years of disease onset [2]. Our observations support that evaluation of dystonia severity alone cannot fully capture the long-term clinical deterioration caused by mixed movement disorders associated with PKAN.
Surgical infection rates were higher in the present study than in the meta-analysis of DBS outcomes in PKAN cases (3 out of 11 vs. 6 out of 99) by De Vloo et al. [11]. The rate was also higher than that reported in a systematic review of DBS complications in dystonia of all causes (44 out of 592) [21]. Our three patients with complications had classic PKAN and severe generalized dystonia, with status dystonicus in one patient who was as young as age 10 at the time of surgery. This corresponds to a meta-analysis that reported more frequent surgical infections in younger patients and those who underwent surgery for status dystonicus [11]. We speculate that the higher average BFMDRS-M scores in our patients with classic PKAN (92.1 vs. 81.6 reported by De Vloo et al. [11]) may have contributed to the higher rates of surgical infections in this study.
A limitation of this study lies in the descriptive nature of the study, with the raters not blinded to the patient’s clinical information, including their previous dystonia scores, which could influence the evaluation of favorable outcomes. In addition, one may argue that patients who responded to long-term follow-up had a relatively favorable prognosis among the entire group of patients. However, all patients who received bilateral GPi-DBS for PKAN in Seoul National University Hospital and Asan Medical Center were clinically evaluated for at least 2 years postoperatively, and no cases were excluded from our analysis. All available descriptive clinical information on adverse events and disease progression was provided within and beyond the duration of the BFMDRS follow-up in all applicable cases. Additionally, for a more comprehensive assessment of the patients’ condition, additional scales evaluating, e.g., pain, cognition, activities of daily life, or psychiatric features, may be applied in future studies.
In conclusion, the present study demonstrates that bilateral pallidal stimulation for dystonia in patients with genetically confirmed PKAN showed a lasting beneficial effect on dystonia for more than 5 years postoperatively. In the absence of a curative treatment for PKAN, our findings support the implementation of GPi-DBS for the management of dystonia in patients with PKAN.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.14802/jmd.22002.
Supplementary Table 1.
Serial BFMDRS scores and detailed clinical outcome after DBS implantation
Supplementary Figure 1.
Flowchart of the study patients. Patients with genetically confirmed PKAN with a clinical diagnosis of neurodegeneration with brain iron accumulation (NBIA) who received bilateral GPi-DBS for moderate to severe medically intractable dystonia were eligible for the study. Patients who were evaluated for BFMDRS-M score by a movement disorder specialist before surgery and had information available regarding clinical status after DBS implantation for ≥ 2 years postoperatively were included. GPi-DBS, globus pallidus internus deep brain stimulation; PKAN, pantothenate kinaseassociated neurodegeneration; SNUH, Seoul National University Hospital; AMC, Asan Medical Center; BFMDRS-M, Burke–Fahn–Marsden Dystonia Rating Scale motor subscale.
Notes
Conflicts of Interest
Kyung Ah Woo, Seung-Ho Jeon, Kye Won Park, Seung Hyun Lee, Hye Ran Park, Jong-Hee Chae, and Sun Ha Paek have nothing to disclose.
Han-Joon Kim received research grants from Seoul National University Hospital, Institute of Information and Communications Technology Planning and Evaluation, Forest Hills, and HLB Pharmaceutical.
Sun Ju Chung was supported by a grant from the Korea Healthcare Technology R & D Project, Ministry of Health & Welfare, Republic of Korea (HI19C0256).
Beomseok Jeon received research grants from Kuhnil Pharmaceutical, Peptron, Novartis Korea, Ipsen Korea, Samil Pharmaceutical, Abbvie Korea, Seoul National University Hospital Research Fund, Seoul National University College of Medicine, and Korean Brain Research Institute.
Funding Statement
None
Author Contributions
Conceptualization: Han-Joon Kim. Data curation: Kyung Ah Woo, Han-Joon Kim, Seung-Ho Jeon, Hye Ran Park, Kye Won Park, Seung Hyun Lee, Sun Ju Chung, Jong-Hee Chae. Formal analysis: Kyung Ah Woo, Han-Joon Kim. Investigation: Kyung Ah Woo, Han-Joon Kim. Methodology: Kyung Ah Woo, Han-Joon Kim. Project administration: Han-Joon Kim. Supervision: Han-Joon Kim. Writing—original draft: Kyung Ah Woo, Han-Joon Kim. Writing—review & editing: Han-Joon Kim, Kye Won Park, Seung Hyun Lee, Sun Ju Chung, Jong-Hee Chae, Sun Ha Paek, Beomseok Jeon.