Association Between Gait and Dysautonomia in Patients With De Novo Parkinson’s Disease: Forward Gait Versus Backward Gait
Article information
Abstract
Objective
Studies on gait and autonomic dysfunction have been insufficient so far, particularly de novo Parkinson’s disease (PD). The aim of this study was to identify the association between gait dynamics and autonomic dysfunction in patients with de novo PD.
Methods
A total 38 patients with de novo PD were retrospectively included in this study. Details of patients’ dysautonomia were assessed using the Scales for Outcomes in Parkinson’s Disease-Autonomic Dysfunction (SCOPA-AUT). For assessment of gait, a computerized gait analysis was performed using the GAITRite system for forward gait and backward gait. High SCOPA-AUT score (PD-HSAS) group and low SCOPA-AUT score (PD-LSAS) group were identified according to their SCOPA-AUT scores.
Results
Nineteen (50%) patients with high SCOPA-AUT scores above median value (12.5) were assigned into the PD-HSAS group and others were assigned to the PD-LSAS group. Compared with the PD-LSAS group, the PD-HSAS group exhibited slower gait, shorter stride, decreased cadence, increased double support phase, decreased swing phase, and increased variability in swing time. Total SCOPA-AUT score showed significantly positive correlations with gait variability and instability but a negative correlation with gait hypokinesia. In subdomain analysis, urinary dysautonomia was highly associated with impairment of gait dynamics. All significant results were found to be more remarkable in backward gait than in forward gait.
Conclusion
Our findings suggest that alteration in gait dynamics, especially backward gait, is highly associated with autonomic dysfunction in patients with de novo PD.
Gait impairment is the most disabling symptom in patients with Parkinson’s disease (PD). This symptom is related to the risk of falling and significantly affects functional activities of daily life and the quality of life (QoL) of patients [1]. Therefore, many previous studies have focused on the prediction and early detection of gait alterations for fall prevention from the early stage of PD [2]. In addition to motor disabilities, a wide range of nonmotor symptoms (NMSs), including sleep disorders, neuropsychiatric symptoms, cognitive impairments, and autonomic dysfunctions, are experienced by patients with PD from the premotor stage to all disease stages. These factors also highly affect the daily life functioning and QoL of patients with PD. In particular, autonomic dysfunction, such as symptomatic orthostatic hypotension and cardiovascular dysfunction, is highly associated with falls and gait impairment in patients with advanced PD [3]. Moreover, recent studies have shown that autonomic dysfunctions, especially genitourinary and gastrointestinal (GI) dysfunctions, are highly associated with fall risk and severe motor disability, such as postural instability and gait difficulty (PIGD), in the early stage of PD [4-6]. However, the characteristics of gait alteration and autonomic dysfunctions in patients with de novo PD have been less investigated to date. The impact of autonomic dysfunction on gait is still underresearched. Moreover, previous studies were mostly based on self-report questionnaires and scales to assess gait impairment and falls. Therefore, detailed investigations using objective measurements for gait dynamics related to autonomic dysfunctions are needed for patients with de novo PD. In addition, analysis of various gait types (forward gait and backward gait) that can better reflect the gait characteristics of patients with de novo PD is necessary for accurate study results.
Therefore, the objectives of this pilot study were 1) to investigate the impact of autonomic dysfunction on gait impairment and 2) to examine the association between gait alteration and various forms of dysautonomia, especially in patients with de novo PD. Gait parameters were analyzed utilizing the computerized GAITRite system for measuring gait dynamics and the Scales for Outcomes in Parkinson’s Disease-Autonomic Dysfunction (SCOPA-AUT) for evaluating autonomic dysfunction.
MATERIALS & METHODS
Participants
This study was approved by the Institutional Review Board of Soonchunyang University Seoul Hospital (approval number: 2021-03-012). We retrospectively enrolled patients with de novo PD who underwent gait analysis using the GAITRite system (CIR System Inc., Franklin, NJ, USA) and the assessment of NMSs using several scales and cognitive tests at the initial evaluation. The patients were registered in our hospital between July 2017 and December 2020. We thoroughly reviewed all medical records of patients, including brain MRI and dopamine transporter imaging (DAT). Initial clinical PD diagnosis was made according to the UK Brain Bank criteria [7]. For strict inclusion and exclusion, all patients were evaluated clinically and radiologically. First, patients with previous stroke, head trauma, coexisting vestibulopathy, diabetes mellitus, or other diseases associated with autonomic neuropathy, musculoskeletal problems, or significantly impaired cognition were excluded from this study for correct gait evaluations. Patients with atypical features, including poor levodopa responsiveness, early frequent falls, abnormal eye movement, symptomatic orthostatic hypotension, or ataxia, were clinically excluded because of the possibility of atypical parkinsonism. In addition, any patients exhibiting atypical features, including cerebellar atrophy, hot cross bun sign, middle cerebellar peduncle sign, putaminal abnormalities, hydrocephalus and stoke lesion on brain MRI, or showing atypical pattern of PD on DAT imaging (i.e., incompatible with the rostrocaudal pattern of striatal dopaminergic loss) were radiologically excluded [8,9]. Finally, a total of 38 patients with de novo PD were enrolled in the present study. The follow-up duration was 2.9 ± 0.4 (mean ± standard deviation [SD]) years, and the minimum follow-up duration was 1.5 years. Until now, all patients were clinically regarded as having PD with good and sustained response to anti-parkinsonian drugs, although we did not compare the detailed changes in motor symptoms.
Clinical assessments
The diagnosis of PD was made by a movement disorder specialist in our clinic for all patients. Baseline demographic information and clinical characteristics, including motor and NMSs, were assessed. Parkinsonian motor symptoms and severity were evaluated using the Unified Parkinson’s Disease Rating Scale (UPDRS) part-III total score, PIGD subscore and the Hoehn and Yahr (HY) stage [10]. NMSs of patients were evaluated using the Korean version of the Montreal Cognitive Assessment (MoCA-K) for global cognitive function and the Korean version of the SCOPA-AUT for autonomic dysfunction [11,12].
The SCOPA-AUT is a self-report questionnaire assessed by each patient. The questionnaire is composed of 25 items including six domains to assess GI (7 items), urinary (UR, 6 items), cardiovascular (3 items), thermoregulatory (4 items), pupillomotor (1 item), and sexual (2 items for males and 2 items for females) functions. The frequency of symptoms is scored from 0 (never) to 3 (often). The total summed score is 69. We assessed the total cumulative scores of all domains and subscores of each affected subdomain.
To evaluate the impact of autonomic dysfunction on gait alteration, we divided patients into two groups: the PD with low SCOPA-AUT score (PD-LSAS) group with scores below the median value and the PD with high SCOPA-AUT score (PD-HSAS) group with scores above the median value based on dichotomized total scores using median values of total SCOPAAUT scores for all patients.
Additionally, we evaluated white matter changes using the modified Fazekas scale based on fluid attenuated inversion recovery (FLAIR) images in each patient with de novo PD [13].
Gait assessments
To assess objective measurements of gait dynamics, the GAITRite system with a 4.6-m-long walkway mat was used. All enrolled patients were required to walk barefoot at a comfortable speed. Forward gait and backward gait were randomly assessed for each patient to lessen possible learning effects. To minimize acceleration/deceleration effects of initiation/termination at usual gait, patients were required to start walking two steps before the mat and stop walking two steps after the mat. Each gait was performed ten times. Individual gait assessment using the GAITRite system was conducted at drug-naïve status. For each cycle, the following spatiotemporal gait parameters were recorded: walking speed (cm/s), cadence (steps/min), stride length (cm), step/stride time (s), single/double support time (% gait cycle [GC]), swing/stance phase (% GC), and step width (cm). The coefficient of variation (CV) for each parameter was calculated as 100 × SD/mean of any given variable.
Statistical analyses
Descriptive statistics are presented as the mean ± SD or frequencies for each clinical characteristic. Comparisons between the PD-LSAS group and the PD-HSAS group of patients with de novo PD were analyzed by the chi-square test for categorical variables or the Mann–Whitney U test for numerical variables. Correlation analyses between gait parameters and SCOPA-AUT scores of patients were conducted using Spearman’s rank correlation coefficient after controlling for age, sex, UPDRS-III total score, MoCA-K and white matter burdens. We excluded the analysis of the sexual domain of the SCOPA-AUT due to many missing values, while other domains of the SCOPA-UAT were completed without missing values. A p value of less than 0.05 was accepted as statistically significant. All statistical analyses in the present study were performed using SPSS version 20.0 (IBM Corp, Armonk, NY, USA).
RESULTS
Comparison of clinical features between the high SCOPA-AUT score group and the low SCOPA-AUT
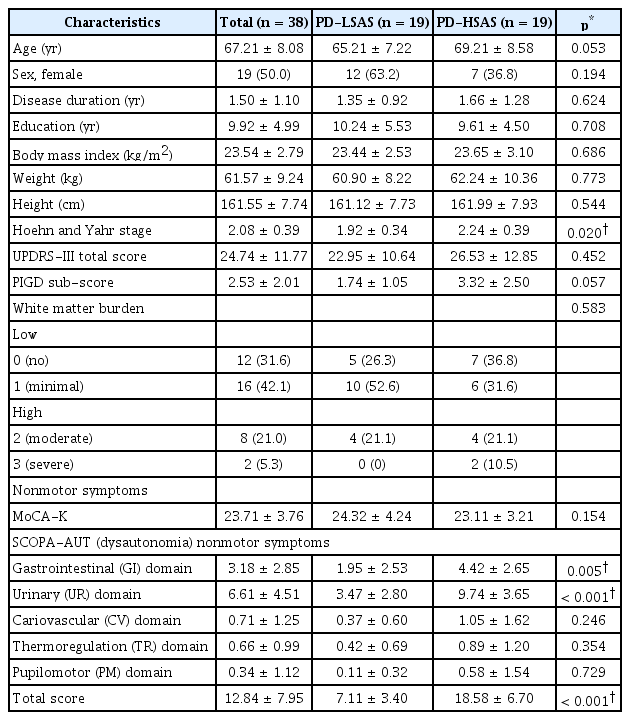
score group of patients with de novo PD The median value of the total SCOPA-AUT for all enrolled patients was 12.5. Out of 38 patients with de novo PD enrolled in the present study, 19 (50%) patients had PD-HSAS above the median value. They were classified into the PD with PD-HSAS (n = 19) group, while others who had scores below the median value were classified into the PD with PD-LSAS (n = 19) group. Clinical demographics and characteristics, including motor and NMSs, especially autonomic dysfunctions, of the two groups are presented in Table 1. Baseline demographics and total scores of UPDRS-III were not significantly different between the two groups. However, the PD-HSAS group had higher (p = 0.020) HY stage scores than the PD-LSAS group.
Demographic and clinical features of de novo Parkinson’s disease patients according to the burden of dysautonomia
To determine differences in various burdens of dysautonomia, SCOPA-AUT scores, including subdomains, were compared between the two groups. Compared to the PD-LSAS group, the PD-HSAS group showed significantly higher scores in the GI domain of SCOPA-AUT (p = 0.005) and UR domain of SCOPAAUT (p < 0.001) (Table 1). In both groups, scores of the UR domain were the highest, followed by scores of the GI domain. There were no significant differences in scores of other subdomains between the two groups.
Comparisons of gait dynamics alterations between the high SCOPA-AUT score group and the low SCOPA-AUT score group with de novo PD
Spatiotemporal gait parameters for two gait types (forward gait and backward gait) in patients with de novo PD divided into two groups (PD-HSAS group and PD-LSAS group) are presented in Table 2. Compared to the PD-LSAS group, the PD-HSAS group showed slower gait (p = 0.005), shorter stride (p = 0.050), decreased cadence (p = 0.008), increased stride time (p = 0.008), increased double support phase (p = 0.003), decreased swing phase (p = 0.015), and increased variability in swing time (p = 0.018) for backward gait. However, only two significant differences were revealed for forward gait in the PD-HSAS group: decreased swing phase (p = 0.027) and increased variability in swing time (p = 0.050).
Correlation of gait features and autonomic dysfunction in patients with de novo PD
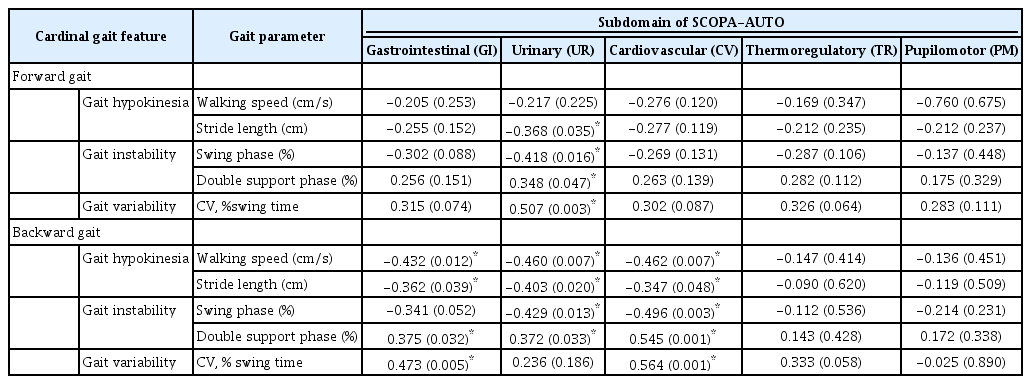
We investigated whether cardinal gait parameters in two gait conditions were correlated with autonomic dysfunctions in patients with de novo PD. As shown in Figure 1, the total SCOPA-AUT score had significantly negative correlations with gait speed (rs = -0.542, p = 0.002) in backward gait and stride length (r = -0.430, p = 0.012; rs = -0.429, p = 0.013) (i.e., gait hypokinesia) in forward gait and backward gait. For the correlation of gait variability (Figure 2), the total SCOPA-AUT score showed a significantly positive correlation with swing time variability (rs = 0.537, p = 0.001; rs = 0.463, p = 0.007) in forward gait and backward gait. For the correlation of gait instability (Figure 3), the total SCOPA-AUT score had negative correlations with swing phase (rs = -0.460, p = 0.007; rs = -0.463, p = 0.007) and positive correlations with double support time (rs = 0.409, p = 0.018; rs = 0.479, p = 0.005) in forward gait and backward gait.
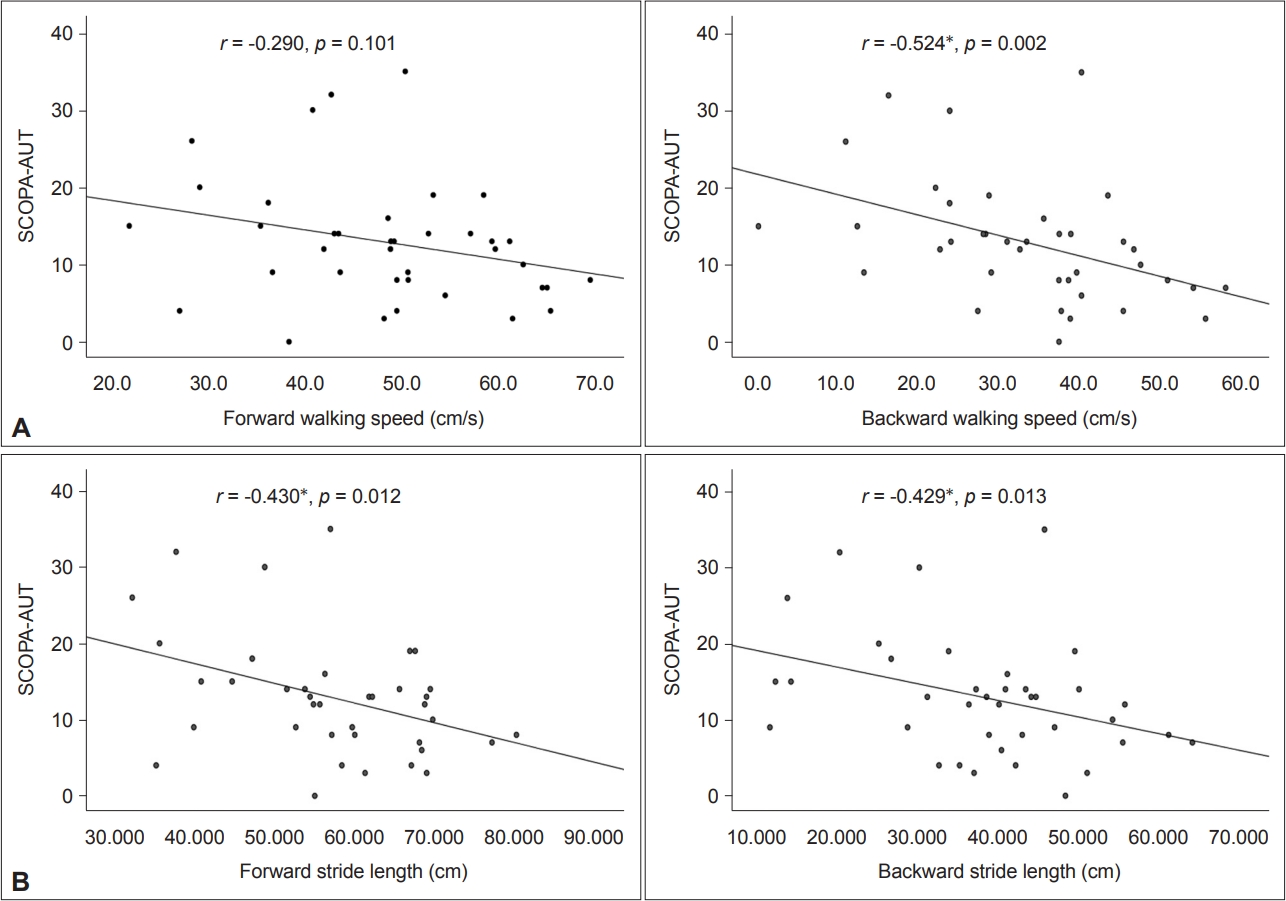
Association between gait hypokinesia and total SCOPA-AUT in patients with de novo PD. A: The total SCOPA-AUT score had no significant correlations with walking speed in the forward gait (rs = -0.290, p = 0.101), but there was a significant positive correlation with walking speed in the backward gait (rs = -0.524, p = 0.002). B: The total SCOPA-AUT score had a negative correlation with stride length (rs = -0.430, p = 0.012; rs = -0.429, p = 0.013) in both the forward gait and backward gait. *p < 0.05. SCOPA-AUT, Scales for Outcomes in Parkinson’s Disease-Autonomic Dysfunction; PD, Parkinson’s disease.

Association between gait variability and total SCOPA-AUT in patients with de novo Parkinson’s disease. The total SCOPA-AUT score showed a significantly positive correlation with swing time variability (rs = 0.537, p = 0.001; rs = 0.463, p = 0.007) in both the forward gait and backward gait. *p < 0.05. CV, coefficient of variation; SCOPA-AUT, Scales for Outcomes in Parkinson’s Disease-Autonomic Dysfunction.
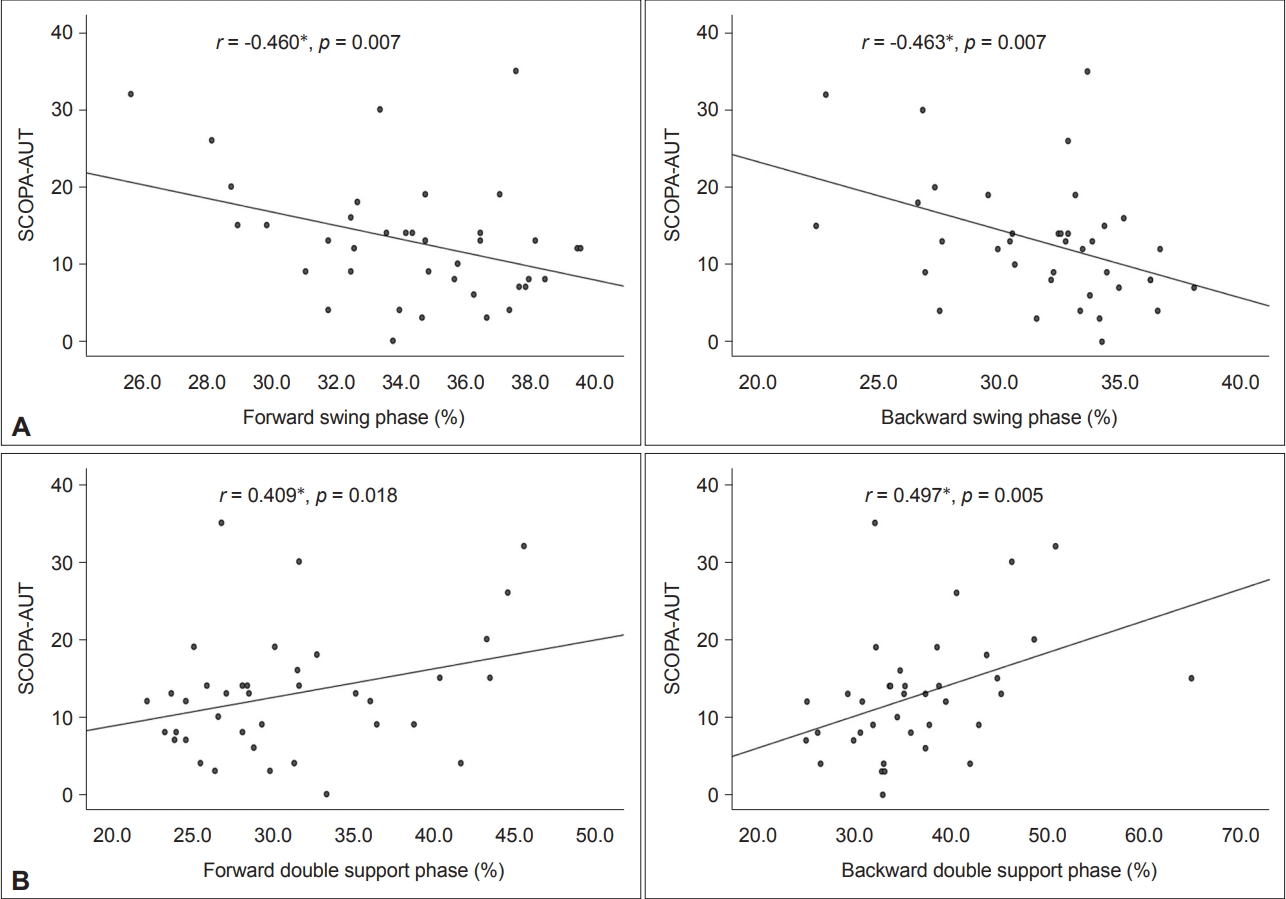
Association between gait instability and total SCOPA-AUT in patients with de novo Parkinson’s disease. A: The total SCOPA-AUT score had a significantly negative correlation with swing phase (rs = -0.460, p = 0.007; rs = -0.463, p = 0.007) in both forward gait and backward gait. B: The total SCOPA-AUT score had a significantly positive correlation with double support time (rs = 0.409, p = 0.018; rs = 0.497, p = 0.005) in both the forward gait and backward gait. *p < 0.05. SCOPA-AUT, Scales for Outcomes in Parkinson’s Disease-Autonomic Dysfunction.
Associations of gait features with various forms of dysautonomias in patients with de novo PD
As a post hoc analysis, we conducted a correlation study between gait parameters and SCOPA-AUT subdomains (Table 3). The subscore of the UR domain showed significantly negative correlations with stride length (rs = -0.368, p = 0.035; rs = -0.403, p = 0.020), swinging phase (rs = -0.418, p = 0.016; rs = -0.403, p = 0.020) and positive correlation with double support phase (rs = 0.348, p = 0.047; rs = 0.372, p = 0.033) in forward gaits and backward gaits. Additionally, swing time variability (rs = 0.507, p = 0.003) had a significantly positive correlation in forward gait, and gait speed (r = -0.460, p = 0.007) had a significantly negative correlation in backward gait with the subscore of the UR domain. In backward gait, subscores in the GI and CV domains were mostly significantly correlated with all gait parameters except swing phase in the GI domain. However, in forward gait, there were no significant results except for the UR domain.
DISCUSSION
In the present study, we investigated drug naïve patients with de novo PD to reveal the association between autonomic dysfunctions and gait dynamics alterations based on two types of walking (forward gait and backward gait). We also assessed correlations between various dysautonomic subdomains and major gait parameters in patients with de novo PD. In the evaluation of differences in gait parameters between the PD-HSAS group and the PD-LSAS group (Table 2), significant differences in gait dynamics between the two groups were not shown in forward gait except for parameters related to swing phase. The PD-HSAS group showed significantly slower walking speed, decreased cadence, and shorter stride but increased stride time than the PD-LSAS group in backward gait. Furthermore, a decreased swing phase, increased double support phase, and increased variability of swing time in backward gait were shown in the PD-HSAS group. On the other hand, the PD-HSAS group showed a decreased swing phase only in forward gait. These results suggest that de novo PD patients with severe autonomic dysfunctions might walk more slowly with short strides and decreased cadence, more inconsistently with variable swings, and more unstably with longer double support, especially when walking backward. Moreover, in the correlation analysis (Figures 1-3), some significant correlations were found in forward gait but accounted for a larger proportion in backward gait. These findings are consistent with the literature showing that backward gait dynamics compared to forward gait dynamics are more closely related to motor symptoms and fear of fall in de novo PD patients [14]. These findings support that backward gait rather than forward gait might more susceptibly reflect the primary function of the basal ganglia in the early stage of PD. Furthermore, this result may represent the possible association between autonomic dysfunction and altered backward gait dynamics in de novo PD. Further clinically relevant studies are necessary to address this issue.
For correlations between the total SCOPA-AUT score and gait parameters reflecting cardinal gait features of PD, gait hypokinesia was negatively correlated with the total SCOPA-AUT score, whereas gait variability had a significantly positive correlation with the total SCOPA-AUT score. Notably, the double support phase showed a significantly positive correlation, while the swing phase showed a negative correlation with autonomic dysfunction. Increased double support time and decreased swing time are associated with gait instability in PD [15]. Peppe et al. [16] explained that increased double support time is attributed to an inability to shift weight and center of gravity in preparation for stepping adequately in PD. In addition, decreased swing time was related to reduced walking speed, reduced stride length, and increased double support time, resulting in instability of gait in PD. That is, gait instability had a significantly positive correlation with the total SCOPA-AUT score. These findings suggest that PD patients with higher SCOPA-AUT scores might have an unstable walk and a high risk of falling from the early stage of PD. According to a recent large multicenter cohort study, the severity of dysautonomia was significantly associated with the PIGD motor phenotype [17]. However, regarding autonomic dysfunction in de novo PD, uniform results have not been obtained [18]. This fact might be due to the heterogeneity of autonomic dysfunction based on a wide range of pathophysiologies involved in several neurotransmitter systems and autonomic nervous systems [17-19]. Moreover, the gait system in PD is highly complex because of the involvement of both dopaminergic and nondopaminergic pathogeneses [20]. Although the pathomechanism of how various autonomic dysfunctions are associated with gait dynamic alterations in patients with de novo PD remains unknown, we can cautiously assume that their pathomechanisms overlap. This needs to be elucidated through continuous detailed studies.
In addition, several related factors may affect the association between autonomic dysfunction and/or gait alterations in PD. Some studies have reported that these burdens, such as white matter changes, reduced DAT uptake, motor subtypes, and cognitive dysfunction, are related to these alterations [21-24]. In this pilot study, we considered some factors (i.e., modified-Fazekas scale for white matter changes, MoCA-K for cognitive function, and UPDRS-III total score for motor burden) and applied them as covariates for post hoc analysis. There were no significant differences in considering factors between the PD-LSAS and PD-HSAS groups and no major changes in the results after considering some added covariates in the post hoc analysis, which might be related to the limitation of the small number of participants. Therefore, further research on these issues with larger sample sizes and precise research designs is needed in the future.
In a very recent report analyzing the impact of motor subtype on NMSs, we found that PIGD score had significantly positive correlations with total SCOPA-AUT and UR domain of SCOPA-AUT in early PD [25]. Consistent with current reports, Pagano et al. [26] reported that worse urinary dysfunction is correlated with greater postural instability in de novo PD. In addition, Malek et al. [17] have shown that UR symptoms such as incomplete emptying are highly correlated with motor severity in early PD. Consistent with these reports, our investigations suggest that UR dysautonomia in de novo PD patients is highly associated with impairment in gait dynamics. Furthermore, it is linked to an increased risk of falls. Urinary dysfunction in PD is thought to occur through a complex pathophysiology. That is, UR dysautonomia is not confined to the degeneration of dopaminergic neurons in the substantia nigra. The dysfunction is also related to pathologic changes of multiple locations in the brain, including the pontine micturition center, midbrain periaqueductal gray matter, ventral tegmental area-mesolimbic dopaminergic fibers, and the higher center for micturition (e.g., the prefrontal cortex, medial superior/middle frontal gyri, anterior cingulate cortex and medial superior/middle frontal gyri, anterior cingulate cortex, and supplemental motor area) [27,28]. Therefore, further well-designed and continued studies are needed to verify clear associations and possible mechanisms between UR dysautonomia and gait impairment.
The present study has the following limitations. First, the current study was designed as a retrospective study. However, we consistently registered patients with de novo PD according to the consensus diagnostic criteria and tightly excluded ambiguity based on historical, clinical, and radiological consensuses. Second, the number of participants with de novo PD was relatively small in a single hospital. Thus, the results should be interpreted with caution. Nevertheless, consistent with previous reports, our pilot study results seem to be appropriate for demonstrating the clinical implication between autonomic dysfunction and gait alterations in patients with de novo PD. Further detailed studies are necessary to address this result. Third, autonomic dysfunctions were investigated based on the SCOPA-AUT survey rather than objective measurements. However, SCOPA-AUT has been widely verified and is a useful measurement tool that reflects autonomic dysfunction in PD patients. Of course, ongoing research including a well-designed objective assessment of autonomic dysfunction, especially UR dysautonomia, will be needed. Last, assessments utilizing the GAITRite system were not performed in real walking conditions. Therefore, we tried to minimize acceleration or deceleration effects of gait and reduce learning effects in the experimental condition as mentioned in the Methods. Nevertheless, our study is noteworthy because we provide an understanding of the severity of autonomic dysfunction, especially UR dysautonomia, as a predictive marker with gait alteration and impairment in the early stage of drug naïve PD.
Conclusion
The present study demonstrates that gait hypokinesia, variability, and instability are highly associated with autonomic dysfunction in patients with de novo PD. In addition, alterations in backward gait dynamics were more closely related to dysautonomia than those in forward gait dynamics. To the best of our knowledge, this is the first study to investigate the association between gait dynamics and autonomic dysfunction via objective gait measurements in de novo PD patients. Our findings suggest that autonomic dysfunction in de novo PD might be a potential predictor of early gait impairment and risk of fall.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.
Funding Statement
This study was supported by the Soonchunhyang University Research Fund (#20220084) (to K-Y Kwon), and this work was supported by the Research Resettlement Fund for the new faculty of the Konyang University Hospital (to S-M Lee).
Author Contributions
Conceptualization: Kyum-Yil Kwon. Methodology: Kyum-Yil Kwon, SeonMin Lee. Data curation: Mina Lee, Eun Ji Lee, Rae On Kim. Formal analysis: Seon-Min Lee. Funding acquisition: Kyum-Yil Kwon, Seon-Min Lee. Investigation: Kyum-Yil Kwon. Supervision: Kyum-Yil Kwon, Yongduk Kim. Writing—original draft: Seon-Min Lee. Writing—review & editing: Kyum-Yil Kwon, Seon-Min Lee.
Acknowledgements
None