Potential Link Between Cognition and Motor Reserve in Patients With Parkinson’s Disease
Article information
Abstract
Objective
To investigate whether there is a link between cognitive function and motor reserve (i.e., individual capacity to cope with nigrostriatal dopamine depletion) in patients with newly diagnosed Parkinson’s disease (PD).
Methods
A total of 163 patients with drug-naïve PD who underwent 18F-FP-CIT PET, brain MRI, and a detailed neuropsychological test were enrolled. We estimated individual motor reserve based on initial motor deficits and striatal dopamine depletion using a residual model. We performed correlation analyses between motor reserve estimates and cognitive composite scores. Diffusion connectometry analysis was performed to map the white matter fiber tracts, of which fractional anisotropy (FA) values were well correlated with motor reserve estimates. Additionally, Cox regression analysis was used to assess the effect of initial motor reserve on the risk of dementia conversion.
Results
The motor reserve estimate was positively correlated with the composite score of the verbal memory function domain (γ = 0.246) and with the years of education (γ = 0.251). Connectometry analysis showed that FA values in the left fornix were positively correlated with the motor reserve estimate, while no fiber tracts were negatively correlated with the motor reserve estimate. Cox regression analysis demonstrated that higher motor reserve estimates tended to be associated with a lower risk of dementia conversion (hazard ratio, 0.781; 95% confidence interval, 0.576–1.058).
Conclusion
The present study demonstrated that the motor reserve estimate was well correlated with verbal memory function and with white matter integrity in the left fornix, suggesting a possible link between cognition and motor reserve in patients with PD.
The concept of motor reserve was recently introduced to explain the presence of individual variability in parkinsonian motor deficits despite the similar degree of nigrostriatal dopamine depletion in patients with Parkinson’s disease (PD) [1-3]. Similar to the concept of cognitive reserve in Alzheimer’s disease (AD) [4], motor reserve reflects an individual’s capacity to tolerate neuropathological lesions in PD [5]. Several studies have reported that educational attainment, which is one of the most representative proxies for measuring cognitive reserve in AD populations [4], is also associated with motor reserve in PD populations [3,6-9]. In contrast to cognitive reserve, individuals with greater motor reserve appear to cope better with neurodegenerative pathology throughout disease progression [2], indicating a passive reserve model for motor reserve in PD [3,9].
Previously, we identified the functional brain network associated with motor reserve, which consisted of the basal ganglia, cerebellar vermis, inferior frontal cortex, amygdala, hippocampus, and insula, in patients with PD [3]. This network shares the core components of the network associated with cognitive function as well as motor function, suggesting a possible link between cognitive function and motor reserve in PD. In fact, there is increasing clinical evidence to support the potential association between cognitive dysfunction and motor disability in patients with PD [10]. In the present study, we first estimated the motor reserve of each patient with newly diagnosed PD using the residual-based approach as described in our previous work [3]. We then performed correlation analyses between the motor reserve estimate and composite score of each cognitive function domain. We also performed a diffusion connectometry analysis to investigate whether white matter (WM) pathways associated with the motor reserve estimates share the substrates related to cognitive function. Additionally, we assessed the effect of initial motor reserve on the risk of dementia conversion in patients with PD.
MATERIALS & METHODS
Participants
We retrospectively reviewed the Yonsei Parkinson Center database medical records of patients with newly diagnosed PD who visited the Movement Disorders outpatient clinic at Yonsei University Severance Hospital between January 2015 and April 2018. PD was diagnosed according to the clinical diagnostic criteria of the UK PD Society Brain Bank. During this time, a total of 163 patients with PD underwent [18F] N-(3-fluoropropyl)-2βcarbomethoxy-3β-(4-iodophenyl) nortropane positron emission tomography (18F-FP-CIT PET), brain magnetic resonance imaging scans available for diffusion tensor imaging (DTI) analyses, and a detailed neuropsychological test at the initial assessment. Patients did not present additional atypical features (e.g., poor response to dopaminergic medications, ataxia, prominent autonomic dysfunction, vertical gaze limitation, early fall, and cortical sensory loss) during the follow-up period (> 3 years). Parkinsonian motor symptoms were assessed using the Unified Parkinson’s Disease Rating Scale Part III (UPDRS-III). The Korean version of the Mini-Mental State Examination (K-MMSE) was used to assess general cognition [11]. Olfactory function and depression were evaluated using the cross-cultural smell identification test and the Beck Depression Inventory (BDI), respectively. The WM hyperintensity severity as seen on fluid-attenuated inversion recovery images was rated using the Scheltens scale [12]. This study was approved by the Yonsei University Severance Hospital Institutional Review Board (4-2020-1449). The need for informed consent was waived because of the retrospective nature of the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards.
Estimation of motor reserve
18F-FP-CIT PET was performed using a Discovery 600 (GE Healthcare, Milwaukee, WI, USA) device, and dopamine transporter (DAT) availability in the putamen was quantified as described previously (Supplementary Material in the online-only Data Supplement) [3]. The motor reserve of each patient was then estimated based on their baseline UPDRS-III score and DAT availability in the posterior putamen [3], using the residual-based approach that has been applied in some previous studies to define the cognitive reserve in AD populations [13,14]. Specifically, we used the general linear model to predict the UPDRS-III score by using age, sex, disease duration, and the natural logarithm of DAT availability in the posterior putamen in 163 patients with de novo PD. The residuals (i.e., differences between the actual and the predicted UPDRS-III scores) in the general linear model were then calculated and standardized as follows: motor reserve estimate = standardized value of (UPDRS-IIIpredicted - UPDRS-IIIobserved). A greater motor reserve estimate indicated that the patient’s actual UPDRS-III score was lower than the predicted score (i.e., higher motor reserve) [3,13,14].
Additionally, patients with PD were classified into the following groups according to the motor reserve estimate: PD patients with high motor reserve (PD-MR-H; motor reserve estimate > 0.5; n = 58) and PD patients with low motor reserve (PD-MR-L; motor reserve estimate < -0.5; n = 33).
Neuropsychological assessment
All patients underwent the Seoul Neuropsychological Screening Battery, a comprehensive Korean language neuropsychological battery of tests (Supplementary Material in the online-only Data Supplement), at the initial assessment. We previously conducted a factor analysis to determine the cognitive profile for four cognitive function domains (visual memory/visuospatial, verbal memory, frontal/executive, and attention/working memory/language) in patients with PD [15]. In this study, we applied the same formula to calculate the composite scores of each cognitive function domain and the global cognitive composite score.
Connectometry analysis
Imaging preprocessing
The DTI data were acquired using the same protocol as that described in our previous studies (Supplementary Material in the online-only Data Supplement) [16] and were preprocessed in the FMRIB Software Library (FSL version 5.0.9; FMRIB, Oxford, UK) as follows. First, we corrected eddy current distortion and head motion for each scan using the eddy current function of FSL, which employs affine registration to the b = 0 image. Individual brain binary masks were then created from the Brain Extraction Tool with a fractional intensity threshold of 0.2 [17]. Since fractional anisotropy (FA) values represent the degree of directional diffusion [18] rather than the mean diffusivity values, which appear to reflect diminished membrane density [19], we generated FA values by DTIFit, which calculates the tensor to be linearly fitted for every voxel inside the brain [20], for connectometry analysis. All DTI images were visually inspected for any signal dropouts and obvious artifacts before and after the preprocessing procedure.
Correlational tractography between fractional anisotropy and motor reserve estimate
To investigate the pathway associated with motor reserve, diffusion connectometry analysis was applied by mapping the correlational tractography to FA values with the motor reserve estimate. The preprocessed diffusion data were reconstructed in the Montreal Neurological Institute space using q-space diffeomorphic reconstruction with a diffusion sampling length ratio of 1.25 with 2-mm isotropic output resolution to obtain the spin distribution function [21]. For connectometry analysis, a nonparametric Spearman partial correlation was performed to depict the correlation of WM pathways with motor reserve. Since the motor reserve estimate was derived from the residual model using age, sex, and disease severity as variables, we did not include any covariates in the correlation analyses. A deterministic fiber tracking algorithm was conducted to select local connectomes exceeding the t-threshold of 2.5 with four iterations of topology-informed pruning for removing spurious connections [22]. A length threshold of 25-voxel distance was used to identify significant tracks. The statistical significance level for whole-brain connectometry results was set at a false discovery ratio (FDR) of p < 0.05 for multiple comparison correction using 5,000 nonparametric permutation tests. The figure of result tracks was generated using DSI Studio (http://dsi-studio.labsolver.org).
Assessment of dementia conversion according to initial motor reserve
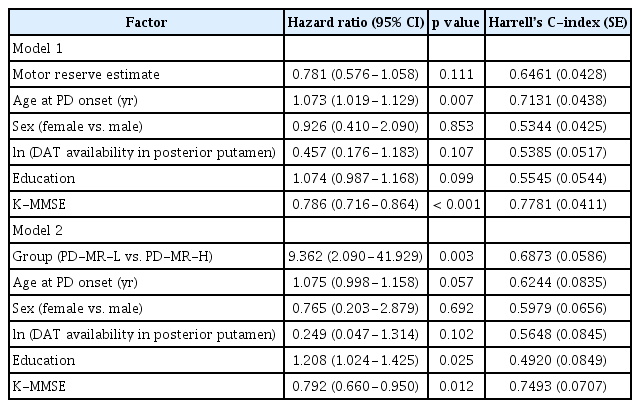
The assessment of dementia conversion during the follow-up period was performed as described in a previous study [15]. After the diagnosis of PD, patients visited the outpatient clinic at 3-month intervals, and two movement disorder specialists assessed the conversion to dementia [23,24]. Survival duration was defined as the time from onset of parkinsonian motor symptoms to the occurrence of dementia or the last clinic visit (for patients without conversion to dementia). A Cox regression model was then used to estimate the hazard ratio (HR) and 95% confidence interval (CI) for the development of dementia according to the initial motor reserve estimate while adjusting for age at PD onset, sex, natural logarithm of DAT availability in the posterior putamen, educational attainment, and K-MMSE scores.
Statistical analyses
To compare the baseline demographic characteristics and cognitive function between the PD-MR-H and PD-M-L groups, the Student’s t test and Pearson’s χ2 test were used for continuous and categorical variables, respectively. Pearson’s correlation coefficient was calculated to assess the relationship between the motor reserve estimate and composite score of each cognitive domain in the 163 patients with PD. A Bonferroni correction was used for multiple testing correction. The time from onset of parkinsonian symptoms to the occurrence of dementia was assessed with Kaplan–Meier estimates in the PD-MR-H and PD-MR-L groups, and a log-rank test was used to compare the Kaplan–Meier plots between both groups. We also compared the dementia-free times between both groups using the Cox regression model, which was adjusted for age at PD onset, sex, natural logarithm of DAT availability in the posterior putamen, educational attainment, and K-MMSE scores. Statistical analyses were performed with SPSS (version 26.0; IBM Corp., Armonk, NY, USA), and results with a two-tailed p value < 0.05 were considered statistically significant.
RESULTS
Baseline clinical characteristics of patients with Parkinson’s disease
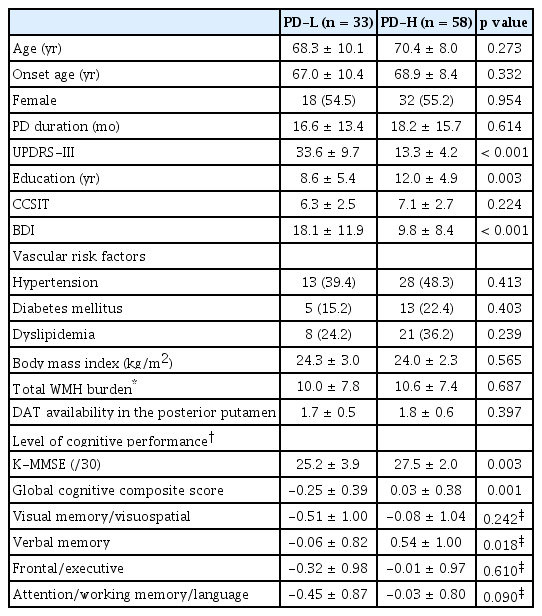
Table 1 shows the baseline clinical characteristics of the 163 patients with newly diagnosed PD. The mean age at onset of PD symptoms was 69.1 ± 8.8 years, and the mean disease duration of PD (i.e., time from symptom onset to diagnosis) was 16.1 ± 13.8 months. The mean UPDRS-III score at the time of PD diagnosis was 21.2 ± 9.5. The average years of education was 10.1 ± 5.2 years, and the mean K-MMSE score was 26.2 ± 3.4.
Estimation of motor reserve
The general linear model demonstrated that predicted UPDRS-III scores were significantly and positively associated with age (β = 0.164, p = 0.046) and disease duration (β = 0.137, p = 0.008) and were negatively associated with the natural logarithm of DAT availability in the posterior putamen (β = -7.823, p < 0.001) (Table 2).
Comparative analyses between Parkinson’s disease groups according to motor reserve estimates
In comparative analyses between the PD groups, the low motor reserve group exhibited greater baseline motor deficits than did the high motor reserve group despite having similar levels of DAT availability in the posterior putamen. There was no significant difference in age, sex, or disease duration between the groups, while patients in the low motor reserve group had a lower level of education (8.6 ± 5.4 years) than did those in the high motor reserve group (12.0 ± 4.9 years; p = 0.003). Patients with low motor reserve had higher BDI scores, lower K-MMSE scores, and lower global cognitive composite scores than did those with high motor reserve (18.1 ± 11.9 vs. 9.8 ± 8.4, p < 0.001; 25.2 ± 3.9 vs. 27.5 ± 2.0; p = 0.003; and -0.25 ± 0.39 vs. 0.03 ± 0.38, p = 0.001, respectively). In particular, the low motor reserve group exhibited a poorer level of cognitive performance in the verbal memory function domain (-0.06 ± 0.82) than the high motor reserve group (0.54 ± 1.00, p = 0.018) (Table 3).
Correlation analyses between cognitive composite scores and motor reserve estimates
Correlation analyses demonstrated that motor reserve estimates were well correlated with years of education (γ = 0.251, p = 0.001), which was previously proposed as a motor reserve proxy [6-9]. Among the four cognitive function domains, motor reserve estimates were positively correlated with the composite scores of the verbal memory function domain (γ = 0.246, p = 0.010) but not with those of other cognitive domains (visual memory/visuospatial function, γ = 0.119, p = 0.774; frontal/executive function, γ = 0.137, p = 0.491; attention/working memory/language function, γ = 0.201, p = 0.061). Motor reserve estimates were also associated with the global cognitive composite scores (γ = 0.237, p = 0.014) (Table 4 and Supplementary Figure 1 in the online-only Data Supplement).
Correlational tractography analyses
Connectometry analyses showed that the FA values in the left fornix were positively correlated with the motor reserve estimate (FDR-corrected p <0.05). There were no fiber tracts in which the FA values were negatively correlated with the motor reserve estimate (Figure 1).
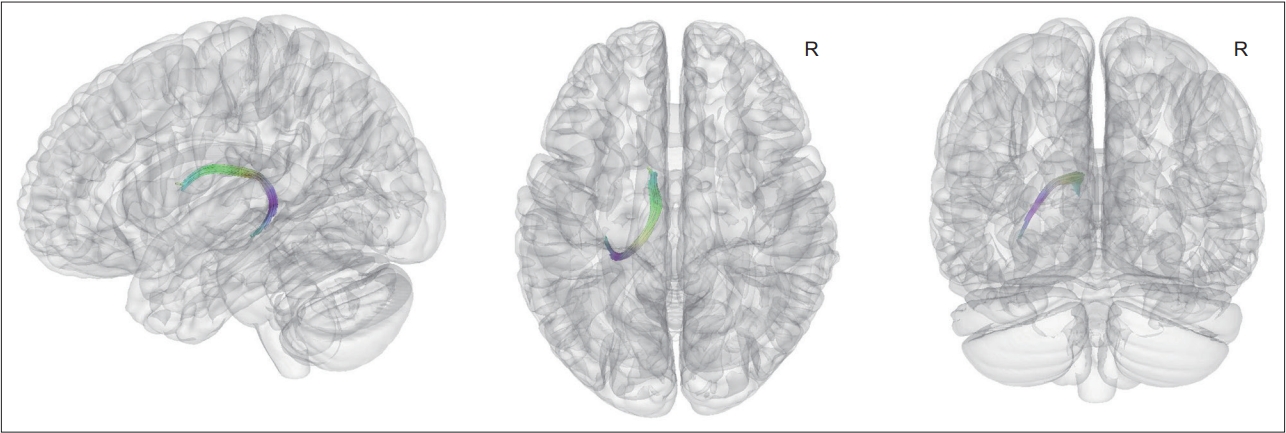
Fiber tracts of which FA values were correlated with motor reserve estimate in patients with newly diagnosed Parkinson’s disease. Connectometry analysis found that FA values of tracts including the left fornix were positively correlated with the motor reserve estimates (false discovery ratio-corrected p < 0.05). There was no significant negative correlation between the motor reserve estimates and the FA values of the fiber pathways. FA, fractional anisotropy.
Initial motor reserve and conversion to dementia
During the follow-up period (4.2 ± 1.6 years), 39 (23.9%) patients with PD developed dementia. Cox regression analysis applied to the entire study cohort demonstrated that higher motor reserve estimates tended to be associated with a lower risk of conversion to dementia (HR, 0.781; 95% CI, 0.576–1.058; p = 0.111) (Table 5), although this result was not statistically significant. In addition, we compared the risk of dementia conversion between the PD-MR-H group and PD-MR-L group to determine whether the trend observed in all 163 patients with PD became significant when comparing both groups. Dementia occurred more frequently in the PD-MR-L group (n = 11, 33.3%) than in the PD-MR-H group (n = 5, 8.6%; p = 0.003). A log-rank test also showed that patients in the PD-MR-L group had a higher risk of dementia conversion than those in the PD-MR-H group (p = 0.008) (Figure 2). The Cox regression model with controlled confounding variables revealed that the PD-MR-L group had a higher risk of conversion to dementia than did the PD-MR-H group (HR, 9.362; 95% CI, 2.090–41.929; p = 0.003) (Table 5). When we calculated Harrell’s C-index for each variable in the Cox regression models, the baseline K-MMSE score was the most important factor for dementia conversion, followed by age at PD onset and initial motor reserve (Table 5).
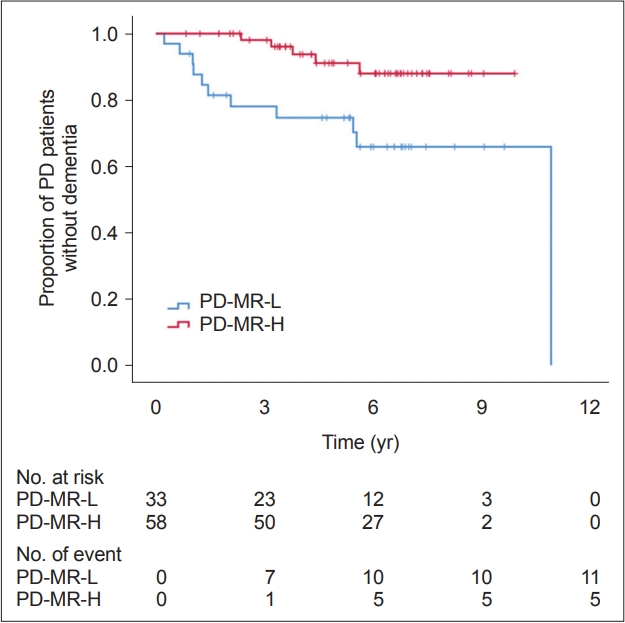
Curves of Kaplan–Meier estimates of the occurrence of dementia after the onset of parkinsonian symptoms in PD-MR-H (n = 58) and PD-MR-L (n = 33). The PD-MR-L group had a higher risk of dementia conversion than did the PD-MR-H group (pLog-rank = 0.008). The crosses in the graphs indicate censored data. PD, Parkinson’s disease; PD-MR-H, PD patients with high motor reserve; PD-MR-L, PD patients with low motor reserve.
DISCUSSION
In the present study, we investigated the association between cognitive function and motor reserve in patients with newly diagnosed PD. The major findings were as follows: 1) Individual motor reserve, which was estimated based on the baseline UPDRS-III scores and DAT availability in the posterior putamen using a residual model, was well correlated with the years of education, a previously reported proxy for motor reserve. 2) The motor reserve estimate was positively correlated with the composite score of the verbal memory function domain and with the global cognitive composite score. 3) Connectometry analysis demonstrated that FA values in the left fornix were positively correlated with the motor reserve estimate, while no fiber tracts were negatively correlated with the motor reserve estimate. 4) Higher motor reserve estimates tended to be associated with a lower risk of dementia conversion during the follow-up period.
Parkinsonian motor symptoms do not appear until nigrostriatal dopamine is 60%–80% depleted. Once the motor signs of PD manifest, there is a wide variability in the severity of motor deficits among patients with PD with similar levels of striatal dopamine loss [1,2,25]. The concept of motor reserve has been proposed to explain this phenomenon, and several compensatory mechanisms, which vary in extent among patients with PD, may work at the nigrostriatal synapses, the extrastriatal basal ganglia output structures, and the motor circuits outside the basal ganglia [3]. Our previous study identified the functional brain network associated with motor reserve in early-stage PD, which was composed of the bilateral basal ganglia (putamen, caudate, and globus pallidus), cerebellar vermis, inferior frontal cortex, amygdala, hippocampus, and the insula [3]. This motor reserve network shared the core components of the network associated with PD and motor function, and this biological relevance strengthened the robustness of the findings. Furthermore, this network also contained substrates related to cognitive function, such as the amygdala, hippocampus, and insula [26], suggesting a possible link between cognitive function and motor reserve in patients with PD.
In this study, the motor reserve estimate was well correlated with WM integrity in the left fornix. In fact, the fornix has axonal connections with the subiculum of the hippocampus [27], which was a component of the functional brain network of motor reserve in our previous study [3]. Moreover, WM disintegration in the fornix is known to be associated with hippocampal damage [28,29] as well as memory impairment in patients with PD [30,31], which is consistent with the correlation analyses showing that the motor reserve estimates were positively correlated with the composite scores of verbal memory function. Moreover, patients in the PD-MR-L group had lower cognitive composite scores in the verbal memory function domain than those in the PD-MR-H group. The association of motor reserve with verbal memory function may also be linked to the finding that only the left side of the fornix was relevant to the motor reserve estimate. Although the exact mechanism remains unclear, there has been increasing evidence to support the link between cognitive dysfunction and motor disability in patients with PD. Anatomical and physiological evidence suggests that both cognitive and motor function can be affected by shared or parallel pathological processes in the cortico-striatal-thalamic loop [32]. Clinical evidence has shown that the presence of cognitive impairment is a predictor of rapid motor decline [10,33-35]. Conversely, axial motor features are relevant to rapid cognitive decline or incident dementia in patients with PD [36,37]. Our finding that the motor reserve estimate was well correlated with the global cognitive composite scores also adds evidence to the parallel development of aberrant corticostriatal plasticity in the cognitive and motor loops [32]. In other words, patients with PD with better cognitive performance, especially in verbal memory function, may have greater neural plasticity or clinical resilience against neurodegenerative pathologies with respect to motor deficits, i.e., greater motor reserve.
We also found that the motor reserve estimate was not correlated with FA values in the WM fiber pathways associated with motor function. One possible explanation for the discrepancy with previous findings [3] is that functional alterations in motor function-related pathways may precede structural WM changes with respect to motor reserve [38]. Alternatively, diffusion connectometry analysis is limited by a relatively low sensitivity to detect WM disruption in short-range fiber pathways, although this drawback is understandable because short-range fiber pathways may affect the spin distribution function values across a small number of voxels [39]. Further studies, using more sophisticated neuroimaging analyses, would be needed to more sensitively identify the WM structural disruption associated with motor reserve.
In terms of cognitive prognosis, greater motor reserve was associated with a lower risk of dementia conversion in patients with PD. Although dementia eventually develops in almost all patients with PD, the timing of dementia onset varies among patients. Our finding supports the concept that patients with high motor reserve would have more efficient or abundant networks to better cope with neurodegenerative processes than those with low motor reserve, thereby having a greater resistance to the development of dementia and motor complications (i.e., passive reserve) [2,3]. This finding also suggests an association between cognitive impairment and motor reserve in PD, which may be mediated by shared substrates related to cognitive function.
Our study had some limitations. First, DAT availability in the posterior putamen may not accurately reflect the extent of nigrostriatal dopaminergic degeneration once nigral cell loss exceeds 50% [40], although other reliable surrogate markers that can replace DAT scans are not yet available. Therefore, it may not be appropriate to assume that motor reserve estimates are associated with the zero-order reaction between DAT binding and parkinsonian motor handicaps. Furthermore, the estimation of motor reserve using the residual-based approach could differ between studies, depending on the dataset. However, we validated the motor reserve estimate by showing a significant correlation with educational attainment, a widely accepted proxy for measuring motor reserve [6-9]. In addition, the motor-symptom laterality was not taken into account when estimating the motor reserve, although it remains unclear whether the UPDRS-III subscores of each side accurately reflect the patients’ disability. Second, the criteria for classifying patients into the PD-MR-H group and PD-MR-L group according to the estimate of motor reserve were somewhat arbitrary. However, the results of the comparative analyses between the two groups were similar to those of the statistical analyses applied to the entire study population. Furthermore, when we classified the patients into the three tertile groups according to the motor reserve estimate, patients in the highest tertile group (i.e., having high motor reserve) still had a lower risk of dementia conversion than did those in the middle and the lowest tertile groups (Supplementary Table 1 and Supplementary Figure 2 in the online-only Data Supplement). Third, we cannot completely rule out the possibility that the individual variability of the severity of parkinsonian motor deficits might be attributed to the coexistence of other neurodegenerative pathologies. In particular, the correlation between the motor reserve estimate and verbal memory function implies a potential role of coexistent AD or Lewy bodies pathology in both motor reserve and verbal memory function in PD. Fourth, the results of diffusion connectometry analysis only showed the relationship between FA values of the fornix and motor reserve but not the possible involvement of other structures important for cognitive performance. Moreover, when we performed a mediation analysis, WM changes in the left fornix did not mediate the association between striatal dopamine deficits and parkinsonian motor symptoms (Supplementary Material, Supplementary Table 2, and Supplementary Figure 3 in the online-only Data Supplement). Finally, further studies on the link between motor reserve and cognition in non-PD populations would provide more robust evidence for our findings.
In conclusion, the results of the present study demonstrated that the motor reserve estimate was associated with verbal memory function and with WM integrity in the left fornix in patients with newly diagnosed PD. These findings suggest a close relationship between motor and cognitive dysfunction in PD in the face of neurodegenerative pathology.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.14802/jmd.22063.
Supplementary Material
Supplementary Table 1.
Cox regression analyses for dementia conversion in PD tertile groups according to motor reserve
Supplementary Table 2.
Relationship between the striatal dopamine loss, white matter change in the fornix, and UPDRS-III scores
Supplementary Figure 1.
Scatter plots of motor reserve estimate and cognitive composite scores.
Supplementary Figure 2.
Curves of Kaplan–Meier estimates of the occurrence of dementia after onset of parkinsonian symptoms PD tertile groups according to motor reserve. During the follow-up period, 5 (9.1%) patients in the highest tertile group (i.e, high motor reserve), 13 (24.5%) patients in the middle tertile group, and 21 (38.2%) patients in the lowest tertile group (i.e., low motor reserve) developed dementia. A log-rank test showed that the highest tertile PD group had a lower risk of dementia conversion than did the other groups (vs. lowest tertile PD group, p = 0.001; vs. middle tertile PD group, p = 0.028). The lowest and middle tertile PD groups had a comparable risk of dementia conversion (pLog-rank = 0.160). The crosses in the graphs indicate censored data. PD, Parkinson’s disease; PD-MR-lowest, the lowest tertile PD group according to motor reserve estimate; PD-MR-middle, the middle tertile PD group according to motor reserve estimate; PD-MD-highest, the highest tertile PD group according to motor reserve estimate.
Supplementary Figure 3.
A mediation analysis for UPDRS-III scores in newly diagnosed PD. The natural logarithm of DAT availability in the posterior putamen and FA values in the left fornix were entered as a predictor and mediator, respectively, in the mediation analysis for the UPDRS-III scores. Age, sex, and disease duration were entered as covariates. Paths that were statistically significant are displayed with unstandardized regression coefficients (β) and SE on solid lines, whereas paths that were not statistically significant are presented as dashed lines. We found that the UPDRS-III scores were directly affected by DAT availability in the posterior putamen (β = -7.807, BootSE = 2.330, p = 0.001), but the effect of DAT availability in the posterior putamen on the UPDRS-III scores was not indirectly mediated by the FA values of the fornix (β = -0.004, BootSE = 0.153, p = 0.979). DAT, dopamine transporter; FA, fractional anisotropy; UPDRS-III, Unified Parkinson’s Disease Rating Scale Part III; BootSE, bootstrapping standard error; PD, Parkinson’s disease.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.
Funding Statement
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number: NRF-2021R1I1A1A01059678) and the Basic Research Lab (BRL) Program (NRF-2020R1A4A1018714).
Author Contributions
Conceptulization: Seok Jong Chung, Yong H. Sohn. Data curation: Seok Jong Chung, Yae Ji Kim, Mijin Yun, Yong Jeong. Funding acquisition: Seok Jong Chung, Yong Jeong. Investigation: Seok Jong Chung, Yae Ji Kim, Mijin Yun, Yong Jeong, Young H. Sohn. Methodology: Seok Jong Chung, Yae Ji Kim, Hye Sun Lee, Mijin Yun, Yong Jeong, Young H. Sohn. Project administration: Yong Jeong, Young H. Sohn. Resources: Mijin Yun, Phil Hyu Lee, Yong Jeong, Young H. Sohn. Supervision: Yong Jeong, Young H. Sohn. Visulaization: Yae Ji Kim, Yong Jeong. Writing—original draft: Seok Jong Chung, Yae Ji Kim, Yong Jeong, Young H. Sohn. Writing—review & editing: Yun Joong Kim, Hye Sun Lee, Mijin Yun, Phil Hyu Lee.