Subjective Cognitive Complaints in Cognitively Normal Patients With Parkinson’s Disease: A Systematic Review
Article information
Abstract
Subjective cognitive complaints (SCCs) refer to self-perceived cognitive decline and are related to objective cognitive decline. SCCs in cognitively normal individuals are considered a preclinical sign of subsequent cognitive impairment due to Alzheimer’s disease, and SCCs in cognitively normal patients with Parkinson’s disease (PD) are also gaining attention. The aim of this review was to provide an overview of the current research on SCCs in cognitively normal patients with PD. A systematic search found a lack of consistency in the methodologies used to define and measure SCCs. Although the association between SCCs and objective cognitive performance in cognitively normal patients with PD is controversial, SCCs appear to be predictive of subsequent cognitive decline. These findings support the clinical value of SCCs in cognitively normal status in PD; however, further convincing evidence from biomarker studies is needed to provide a pathophysiological basis for these findings. Additionally, a consensus on the definition and assessment of SCCs is needed for further investigations.
INTRODUCTION
Parkinson’s disease (PD) is characterized by motor symptoms. However, nonmotor symptoms also affect the daily activities of patients with PD. Cognitive impairment is very common and one of the most disabling nonmotor symptoms in patients with PD [1]. The prevalence of dementia in PD is estimated to be 30%–40% [2,3], and dementia eventually develops in 80% of patients [4]. The emergence of the concept of mild cognitive impairment (MCI) in PD (PD-MCI) as a predementia status [5] has led to the widely accepted diagnostic criteria for PD-MCI proposed by the Movement Disorder Society (MDS) Task Force [6].
Since early detection and diagnosis of MCI are important in managing cognitive impairment, attention to the pre-MCI stage has also increased [7,8]. Since, by definition, the pre-MCI status should include no objective cognitive impairment [9,10], researchers have focused on subjective feelings of cognitive decline. In fact, concern about changes in cognition reported by either the patient or informant is already one of the essential criteria for the diagnosis of MCI due to both Alzheimer’s disease (AD) and PD [6,11]. Subjective feelings about cognitive decline, herein referred to as subjective cognitive complaints (SCCs), exist at all cognitive levels, including normal cognition, MCI, and even dementia [12-14], and SCCs are known to be correlated with objective cognitive deteriorations in nondemented individuals with or without PD [13-18].
In AD spectrum disorders, the characteristics and predictive values of SCCs with normal cognition have been investigated for a long time, but there is a wide range of diversity in the terminology, definition, and assessment of SCCs. In 2014, a conceptual framework for SCCs was introduced, which unified the different terminologies used for indicating SCCs in preclinical AD into “subjective cognitive decline” (SCD) [10]. Currently, SCD is considered a potential at-risk status of AD, and the concept of SCD has come to the forefront in AD research.
Studies presenting results for SCCs in PD have been rapidly increasing; however, the methodologies for defining SCCs, selecting participants, and interpreting results have shown substantial inconsistencies. In particular, many studies have not distinguished SCCs in normal cognition from those in PD-MCI or PD with dementia [19-21]. This review aims to provide an overview of how SCCs in cognitively normal patients with PD have been defined and assessed and to examine the association between SCCs and objective cognitive performance. This review also explores the clinical significance of SCCs supported by their predictive power for subsequent cognitive decline and their association with biomarkers relevant to cognitive impairment.
METHODS
Search strategy
Using the electronic databases of PubMed, Scopus, Web of Science, and Embase, we searched for original articles published prior to March 31, 2022. The search was conducted in titles and abstracts using the following terms: “Parkinson’s disease” combined with either “cognitive complaints,” “memory complaints,” “subjective cognitive,” or “subjective memory.” Furthermore, relevant articles were identified from the reference lists of the selected articles or other sources. Articles not written in English were excluded from this study.
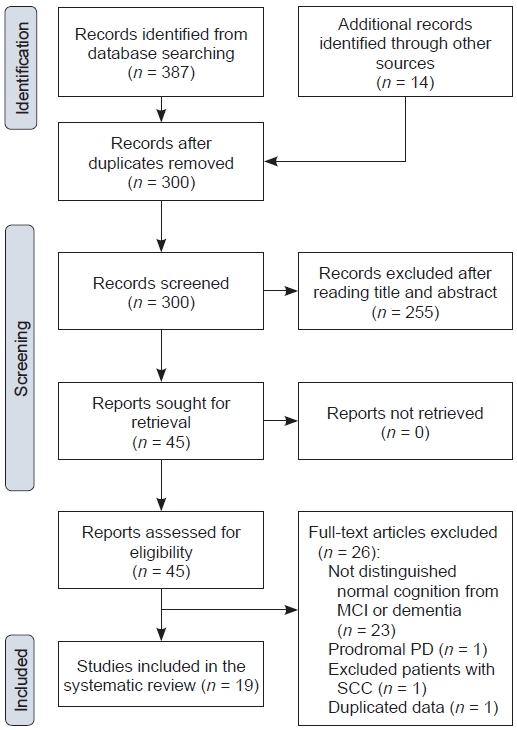
The search yielded 387 articles, and 14 articles were collected from other sources. After removing duplicates, 300 articles were identified.
Selection of studies
The studies were selected by JYH according to the following criteria: 1) studies were conducted in patients with PD; 2) normal cognition was defined using the diagnostic criteria for PD-MCI [6] proposed by the MDS Task Force; 3) methods for SCC assessment were described; 4) SCCs were reported by patients; and 5) data and results for cognitively normal patients with SCCs could be distinguished from those for cognitively impaired patients. Three hundred identified articles were screened, and 255 articles were excluded based on a review of the title and abstracts. The full text of the remaining 45 articles was then read and assessed for eligibility. Among them, 26 articles were excluded for the following reasons: 23 did not distinguish normal cognition from MCI or dementia, one evaluated individual with prodromal PD, one excluded patient with SCC, and one used duplicated data. Finally, 19 articles were included in this review. The selection process is illustrated in Figure 1.
RESULTS AND DISCUSSION
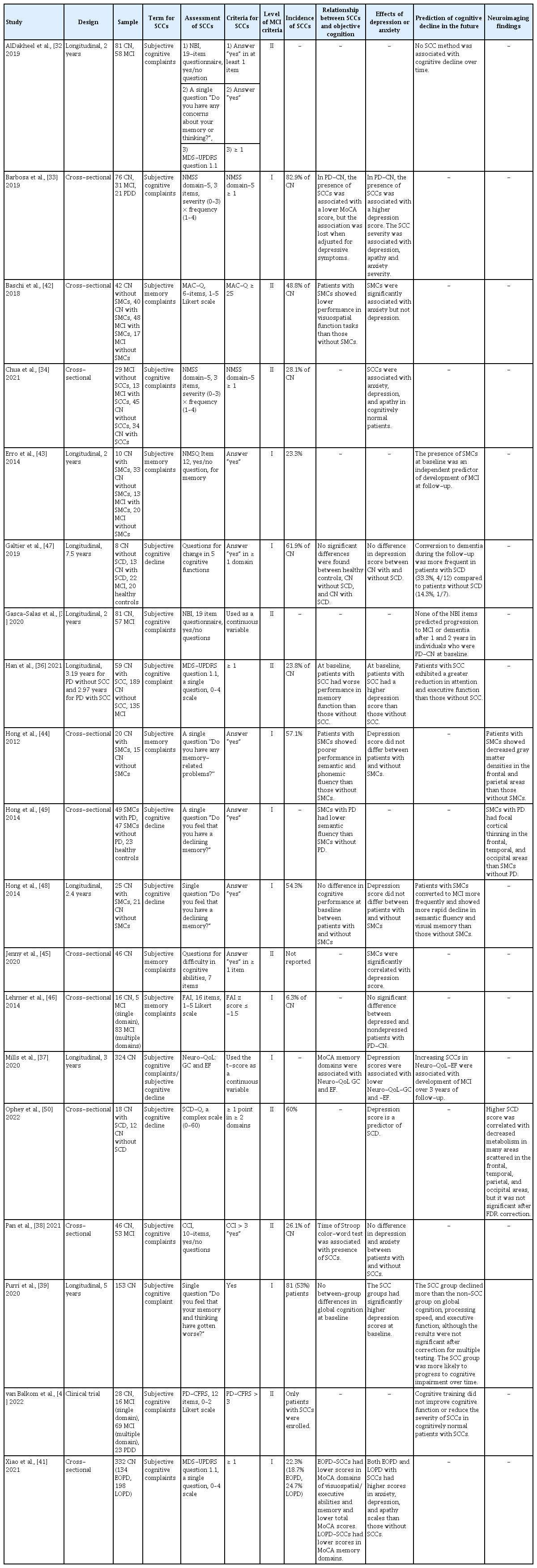
Ten studies were cross-sectional observational studies, and eight studies had a longitudinal observational design (Table 1). The interval between baseline and follow-up in the longitudinal studies ranged from 2 to 7.5 years (mean, 3.3 years). Additionally, one randomized controlled study tested whether cognitive training improves cognitive function.
Terminologies for subjective cognitive complaints
Various terms have been used to indicate SCCs [22], which were originally introduced in the AD spectrum; hence, the subjective feeling of memory impairment was considered the preclinical stage of MCI due to AD [23-28]. However, the conceptual framework proposed by the SCD Initiative Working Group does not restrict subjective impairment to the memory domain [10]. Cognitive impairment in PD also affects multiple cognitive domains [29-31], and the term “cognitive” is recommended to indicate subjective impairment. In the selected studies of this review, the term “SCCs” was the most frequently used (10 studies) [32-41], and “subjective memory complaints (SMCs)” [42-46] and “SCD” [37,47-50] were both used in five studies each. Mills et al. [37] used both “SCCs” and “SCD.” Early studies used the term “memory,” [43,44,46] which seems to be influenced by the term SMCs from the AD field. However, since 2019, when studies on SCCs began to expand, all studies assessed diverse cognitive functions to define SCCs and used the term “cognitive” to indicate SCCs. One study that used “memory” also assessed cognitive functions, including memory; hence, it should have used the term “cognitive.” [45] The term “complaints” (15 times) was more frequently used than “decline” (five times), but there is no consensus on which is more appropriate. Self-perceived cognitive impairment has been studied at all cognitive levels, including MCI and dementia [13,14,17,33,51]. In studies of SCCs in PD, SCCs do not refer to a particular cognitive level but to a subjective symptom [32-35,38,42,43,46]. Therefore, when indicating SCCs in patients with PD, the cognitive level of the patients must be determined. Meanwhile, since a conceptual framework for SCD in preclinical AD was introduced, SCD is generally recognized to indicate a status affiliated with the preclinical stage of AD rather than a symptom, even if there is no expression of “pre-MCI.” [9,10] Since unification in terminology and concepts is important to set further research targets, a consensus for terminology and definition of SCCs in PD is needed.
Assessment of subjective cognitive complaints
Most studies assessed SCCs using a single question or questionnaire. Eight studies determined the presence of SCCs through a single question regarding participants’ memory [32,43,44,48,49] or general cognition [32,36,39,41]. Among them, four studies assessed SCCs using part of a large scale: the Nonmotor Symptoms Questionnaire [52] Item 12 [43] and the MDS-Unified PD Rating Scale (UPDRS) [53] question 1.1 [32,36,41]. Two studies used questions on patients’ performance in five [47] or seven [45] cognitive domains. Ten studies assessed SCCs using questionnaires administered to patients: Non-Motor Symptoms Scale for Parkinson’s Disease [54] domain 5 (two studies) [33,34], Memory Complaints Questionnaire [55] (one study) [42], Neurobehavioral Inventory (NBI) [56] (two studies) [32,35], Quality of Life in Neurological Disorders [57]- Cognition: General Concerns and Cognition: Executive Function (one study) [37], Forgetfulness Assessment Inventory [58] (one study) [46], Subjective Cognitive Decline Questionnaire (one study) [50], Cognitive Complaint Interview [59] (one study) [38], and Parkinson’s Disease Cognitive Functional Rating Scale [60] (one study) [40]. One study simultaneously examined the relationship between SCC and cognitive performance through NBI, a single question, and MDS-UPDRS question 1.1 [32]. To simplify the quantification of SCCs, several questionnaires for SCCs have recently been validated in patients with PD [13,14,16,60,61]. However, there is no evidence to support the superiority of the questionnaire over a single question. AlDakheel et al. [32] explored the predictive ability of three methods, but they failed to reach a conclusion because none of the methods predicted cognitive decline over time. Moreover, many questionnaires assess difficulties in daily activities due to cognitive dysfunction [14,51,60,62], which is similar to the method used to assess functional decline in cognitively impaired patients. Therefore, further research and discussion are needed to answer questions about the proper approach to assess SCCs and the more relevant aspects of the clinical implications of SCCs.
Frequency of subjective cognitive complaints
Thirteen studies provided frequency data. The studies included 438 patients with SCCs (36.3%) out of 1,207 cognitively normal patients. The reported frequencies varied widely, ranging from 6.3% to 82.9% (median, 48.8%).
Objective measurements for cognition
Details of the neuropsychological assessments performed in the included studies are summarized in Table 2. All studies assessed global cognition using the Mini-Mental State Examination (MMSE) [63], Montreal Cognitive Assessment (MoCA) [64], Parkinson Neuropsychometric Dementia Assessment (PANDA) [65], Scales for Outcomes in Parkinson’s Disease–COGnition (SCOPA-COG) [66], and Mattis Dementia Rating Scale-2 (MDRS-2) [67]. Seven and four studies used only the MMSE or MoCA, respectively, and three studies adopted both the MMSE and MoCA. SCOPA-COG was used in two studies, and MDRS-2 and PANDA were used in one study each. SCOPA-COG, MDRS-2, and PANDA were used combined with MMSE or MoCA.
Sixteen studies measured objective cognition using a comprehensive neuropsychological battery. Among them, nine studies involved at least two tests for each of the five cognitive domains according to the recommendation for the level II criteria of PD-MCI. Jenny et al. [45] excluded patients in whom MCI was diagnosed according to the level II PD-MCI criteria, but the details of the neuropsychological assessment were not described in their report. Two studies identified cognitive domains using MoCA items [37,41], and Barbosa et al. [33] selected cognitively normal patients based only on the MoCA total score.
Association between subjective cognitive complaints and objective cognitive performance
SCCs are considered a potential risk factor for future cognitive decline, and many researchers have speculated about subtle cognitive decline in cognitively normal patients with SCCs, even when they show normal performance on standardized cognitive tests. Eleven of the selected studies reported a relationship between SCCs and objective cognitive function [33,36-39,41,42,44,47-49]. In six studies, patients with SCCs showed lower performance in visuospatial function [41,42], verbal fluency [44], inhibitory control ability [38], and memory function [36,37,41] in comparison with patients without SCCs. However, no significant differences between patients with and without SCCs were found in three studies [39,47,48]. In one study, the presence of SCC was associated with a lower MoCA score, but this association was lost when adjusted for depressive symptoms [33]. Hong et al. [49] also reported lower semantic fluency in PD patients with SCCs than in non-PD individuals with SCCs. It remains unclear whether there is minimal objective cognitive decline in cognitively normal patients with SCCs; however, it is noteworthy that three [36,37,41] of four studies with large sample sizes (n > 150) [36,37,39,41] commonly reported lower memory function in participants with SCCs than in those without SCCs.
The predictive value of subjective cognitive complaints for future cognitive decline
To demonstrate that SCCs can predict future cognitive deterioration, it is important to prove their clinical significance. Eight studies investigated whether SCCs are associated with future cognitive decline. Among them, five studies reported that SCCs were predictive of subsequent cognitive impairment. Patients with SCCs exhibited a greater reduction in attention [36,39], executive function [36,39,48], and memory function [48] than those without SCCs. The presence of SCCs at baseline was associated with more frequent development of MCI at follow-up assessments in the 2-year [43], 2.4-year [48], and 3-year [37] longitudinal observations. Purri et al. [39] demonstrated that patients with baseline SCCs were more likely to progress to MCI or dementia in a 5-year follow-up study. Galtier et al. [47] also reported that conversion to dementia was more frequent in patients with SCCs (4 of 12) than in those without SCCs (1 of 7), but the difference was not statistically significant due to the small sample size. However, two studies sharing cohort data showed conflicting results. AlDakheel et al. [32] assessed SCCs using three tools (NBI, single question, and MDS-UPDRS question 1.1), and none predicted cognitive decline. Moreover, none of the NBI items predicted the development of cognitive impairment in cognitively normal patients at baseline [35]. Although diversity in the measurement of SCCs, follow-up duration, assessment of cognitive performance, and sample size might have contributed to the conflicting results, the consistent results justify the significance of SCCs. Therefore, research focusing on defining and assessing SCCs for the best prediction of future decline is expected to be planned in the future.
Association between subjective cognitive complaints and psychiatric problems
Since the development of the concept of SCCs, the possibility that SCCs are another facet of depression has been debated. Memory complaints are a symptom of depression [68], which is common even in the early stages of PD [69]. Therefore, many studies on SCCs in PD have assessed depressive symptoms. Eight of the selected studies reported that SCCs were associated with depression [33,34,36,37,39,41,45,50], whereas six studies did not observe a significant association [38,42,44,46-48]. In particular, two studies reported that the impact of SCCs on lower cognitive performance [33] and faster cognitive decline [39] disappeared when adjusted for depressive scores. Although the association between depression and SCCs remains controversial, it has been suggested that depression is worth assessing in all research on SCCs in patients with PD.
Other psychiatric problems, including anxiety and apathy, also occur frequently in PD [70,71]. Anxiety and apathy were less frequently explored but appeared to be related to SCCs in most studies. Four studies reported an association between SCCs and anxiety [33,34,41,42], whereas two studies did not [38,45]. Only three studies [33,34,41] assessed apathy, but all reported a significant association between apathy and SCCs [33,34,40,41]. In addition, they related to SCCs in the same way as depression in four [33,34,38,41] out of six studies [33,34,38,41,42,45]; therefore, whether anxiety and apathy are independent of depression should be explored.
Biomarkers for subjective cognitive complaints
Biomarkers can provide convincing evidence that SCCs are associated with pathological changes in PD or other coexisting conditions. However, only three neuroimaging studies have been conducted in this regard. The first voxel-based morphometry study demonstrated decreased gray matter density in the frontal and parietal areas and poorer performance on verbal fluency and attention tasks in cognitively normal patients with SMCs than in patients without SMCs [44]. Another study reported that cognitively normal PD patients with SMCs showed less cortical thickness in the frontal, parahippocampal, and occipital areas and poorer performance in semantic fluency than cognitively normal nonPD subjects with SMCs [49]. A recent positron emission tomography study found a correlation between higher SCC scores and decreased metabolism in the right angular gyrus, bilateral middle temporal gyrus, bilateral occipital regions, and left middle frontal gyrus [50]. However, the significance of all three studies was observed in the uncorrected analyses and disappeared after false discovery rate correction. The observed changes in patients with SCCs seem to be related to PD-specific features; however, it is not evident that SCCs reflect a pathological burden related to cognitive impairment in PD.
Many biomarkers are linked to cognitive impairment in PD, including genetic mutations or variants in APOE [72,73], GBA [74,75], MAPT [76], amyloid beta 1–42 concentration in the cerebrospinal fluid [77,78], and positron emission tomography imaging findings for abnormal protein aggregation [79-81]. The concept of SCCs in PD as a prodromal symptom of cognitive impairment is controversial; therefore, research demonstrating a significant association between SCCs and these biomarkers could justify the clinical importance of SCCs.
Clinical trials for subjective cognitive complaints
Only one clinical trial has been conducted on patients with SCCs [40], and the trial aimed to assess the efficacy of cognitive training on cognitive function in patients with PD. Patients with SCCs, regardless of their cognitive level (n = 140), were enrolled and randomized. The experimental and active control groups were trained for eight weeks using online-based cognitive training or nonspecific cognitive engagement, respectively. The results showed no group differences in the Tower of London accuracy in both analyses with the entire population and the cognitively normal subgroup (15 cognitive training and 13 active control).
CONCLUSION
The techniques used for the assessment of SCCs show a wide range of methodological differences. Accordingly, the frequency of SCCs has varied, and the results from previous studies have been inconsistent. This inconsistency has been a major obstacle to the accumulation of evidence and reproduction of reported results. Thus, there is a clear need to reach a consensus on the definition and assessment of SCCs in cognitively normal PD patients.
Previous studies have reported relatively low performance in particular cognitive domains in cognitively normal patients with SCCs; however, the actual association of SCCs with subtle cognitive impairments remains unclear. Meanwhile, the presence of SCCs at baseline is related to faster cognitive decline or more frequent conversion to MCI or dementia in most studies, which strongly suggests the clinical importance of SCCs in the preclinical stage of cognitive decline. Only a few biomarker studies have described the organic changes relevant to cognitive impairment in PD. The accumulation of data from biomarker studies will provide powerful evidence for the existence of SCCs and reveal the pathophysiological characteristics of SCCs.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.
Funding Statement
This work was supported by a grant from the Korea Health Technology R&D Project through the Korean Healthy Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number HU21C0053).
Author Contributions
Conceptualization: Jin Yong Hong, Phil Hyu Lee. Data curation: Jin Yong Hong. Formal analysis: Jin Yong Hong. Funding acquisition: Phil Hyu Lee. Investigation: Jin Yong Hong, Phil Hyu Lee. Methodology: Jin Yong Hong. Supervision: Phil Hyu Lee. Writing—original draft: Jin Yong Hong. Writing review & editing: Phil Hyu Lee.