Safinamide as an Adjunct to Levodopa in Asian and Caucasian Patients With Parkinson’s Disease and Motor Fluctuations: A Post Hoc Analysis of the SETTLE Study
Article information
Abstract
Objective
Safinamide is a selective, reversible monoamine oxidase B inhibitor with demonstrated efficacy and tolerability in placebo-controlled studies and is clinically useful for patients with motor fluctuations. This study evaluated the efficacy and safety of safinamide as a levodopa adjunct therapy in Asian patients with Parkinson’s disease.
Methods
Data from 173 Asian and 371 Caucasian patients from the international Phase III SETTLE study were included in this post hoc analysis. The safinamide dose was increased from 50 mg/day to 100 mg/day if no tolerability issues occurred at week 2. The primary outcome was the change from baseline to week 24 in daily ON-time without troublesome dyskinesia (i.e., ON-time). Key secondary outcomes included changes in Unified Parkinson’s Disease Rating Scale (UPDRS) scores.
Results
Safinamide significantly increased daily ON-time relative to placebo in both groups (least-squares mean: 0.83 hours, p = 0.011 [Asians]; 1.05 hours, p < 0.0001 [Caucasians]). Motor function relative to placebo (UPDRS Part III) improved significantly in Asians (-2.65 points, p = 0.012) but not Caucasians (-1.44 points, p = 0.0576). Safinamide did not worsen Dyskinesia Rating Scale scores in either subgroup, regardless of the presence or absence of dyskinesia at baseline. Dyskinesia was largely mild for Asians and moderate for Caucasians. None of the Asian patients experienced adverse events leading to treatment discontinuation.
Conclusion
Safinamide as a levodopa adjunct is well tolerated and effective in reducing motor fluctuations in both Asian and Caucasian patients. Further studies to investigate the real-world effectiveness and safety of safinamide in Asia are warranted.
The dopamine precursor levodopa has long been established as the gold standard of treatment for patients with Parkinson’s disease (PD). However, high doses of levodopa, long-term use and PD progression are associated with the onset of motor fluctuations such as wearing-off and dyskinesia, complications highly prevalent in patients with advanced PD [1-4]. Such fluctuations cause significant quality-of-life (QoL) impairments, and managing symptoms without worsening motor complications remains a major unmet need for patients with PD [5,6].
Safinamide is a selective, reversible monoamine oxidase B (MAO-B) inhibitor used as an adjunct to levodopa [7]. It has sodium-channel inhibitory effects and prevents stimulation of glutamate release in the basal ganglia [7,8]. Safinamide has been examined in several trials, including international Phase III studies (Study 016 [with 18-month placebo-controlled extension, Study 018], SETTLE), a Japanese Phase II/III study (ME2125-3) and a Chinese Phase III study (XINDI) [9-13]. These trials demonstrated that 50 and 100 mg/day doses of safinamide are effective in improving wearing-off and motor symptoms in patients with PD [9-12]. Safinamide is classified as “efficacious” and “clinically useful” in the International Parkinson and Movement Disorder Society’s recent evidence-based review [14]. It is licensed as add-on therapy to levodopa for patients with PD and wearing-off symptoms in Europe, North America and several Asian countries [15].
However, PD progression and symptoms vary for patients from different countries. Population differences can be partly attributed to biological factors such as genetic polymorphisms [16], which may contribute to heterogeneity in disease progression and response to medication [17,18]. Racial differences in body weight, comorbidities (e.g., hypertension, type II diabetes) and other medication use also affect PD progression, and types of PD symptoms vary between populations [19,20].
Despite the large potential for differences in PD progression, medication response, and clinical practices for patients in Asia, major clinical trials in PD have not studied differences in efficacy and safety for Asian PD patients. Indeed, Asian representation in such trials has generally been poor, as European and US populations have historically been the focus [5,21]. Although other studies have investigated safinamide use in Asians, patients from only one Asian country were enrolled in each study (e.g., Study 016, India; ME2125-3, Japan) [9,11]. As such, data on the efficacy and safety of safinamide in Asian populations are scarce, although such data would help inform clinical decisions in the region.
In the absence of prospective trials examining differences in response between Asian and Caucasian patients with PD, we conducted a post hoc analysis of data from the Phase III Safinamide Treatment as Add-on to Levodopa (SETTLE) study (NCT00627640). SETTLE was an international, double-blind, randomized controlled trial of safinamide as an adjunct to levodopa in patients with motor fluctuations [12]. A substantial proportion of the patients included (approximately 30%) came from the Asia Pacific, including Hong Kong, India, Korea, Malaysia, Singapore, Taiwan, and Thailand [12]. While the SETTLE study was not designed to explore differences in the efficacy or safety of safinamide between various ethnic groups, it nonetheless provides a good opportunity to investigate the effects of safinamide in Asian patients across the region and in Caucasian patients from Europe and North America (Supplementary Table 1 in the online-only Data Supplement) [12]. Such an analysis would provide information for further investigations of the efficacy and safety of safinamide in the under researched Asian population.
MATERIALS & METHODS
Study design and population
Full details of the SETTLE study design and eligibility criteria have been reported [12]. In total, 549 patients were enrolled from 119 centers across 21 countries in Europe, Asia Pacific, and North America between March 5, 2009, and February 23, 2012.
Patients were randomized 1:1 to receive either adjunctive safinamide or placebo. The 50 mg/day starting dose of safinamide was increased to 100 mg/day by Day 14 if no tolerability issues were reported. Enrolled patients were aged 30–80 years and had a diagnosis of idiopathic PD according to clinical evaluation and Queen Square Brain Bank criteria. Patients had been diagnosed for more than 3 years, experienced ‘OFF-time’ of > 1.5 hr per day (excluding morning akinesia), and had a Hoehn and Yahr rating of stages 1–4 during an OFF-phase. Patients were levodopa responsive and following an oral levodopa regimen that had been stable for 4 weeks. Patients with severe, disabling peak-dose or biphasic dyskinesia, wide or unpredictable symptom fluctuations, dementia, cognitive dysfunction, psychosis, or depression were excluded.
Patients from Asian and Caucasian racial subgroups were included in this post hoc analysis. Almost all ethnically Asian patients were enrolled from centers in Asia; only five were enrolled from non-Asian sites (i.e., Western Europe and North America).
The study was conducted in accordance with the Declaration of Helsinki (2013), the International Conference on Harmonization’s Harmonized Tripartite Guideline for Good Clinical Practice, and local laws; and was approved by independent ethics committees and health authorities in all participating countries. All patients provided written informed consent to participate.
Outcomes and evaluations
The primary efficacy outcome was change from baseline to week 24 in mean daily ON-time without troublesome dyskinesia (hereafter, ‘ON-time’), as recorded by patients/caregivers in a diary. Key secondary outcomes were mean change in daily OFF-time (based on diary entries), mean change in Unified Parkinson’s Disease Rating Scale (UPDRS) Part III scores (motor examination), mean change in UPDRS Part II scores (activities of daily living [ADL]) during an ON-phase, proportion of patients with improved Clinical Global Impression (CGI) rating, and mean change in 39-Item Parkinson Disease Questionnaire (PDQ-39) summary index scores. Other secondary outcome measures were based on the UPDRS (Part IV), Patient Global Impression – Change ratings, EuroQoL Five Dimensions (EQ-5D) scores, change in levodopa daily dosage, Dyskinesia Rating Scale (DRS) scores, and Cogtest PD Battery scores.
Safety measures included treatment-emergent adverse events (TEAEs), serious adverse events (AEs), and discontinuations attributed to TEAEs. Impulse-control disorders were assessed by the Questionnaire for Impulsive-Compulsive Disorders in Parkinson Disease (QUIP), and daytime sleepiness was assessed by the Epworth Sleepiness Scale (ESS). The present study also investigated the severity of dyskinesia in Asian and Caucasian subgroups.
Statistical methods
The present study is a post hoc analysis of the SETTLE study, so its statistical methods were not prespecified in the original analysis. All tests were conducted at a significance level of 5% (two tailed), and no adjustments were made for multiplicity. Analyses were performed using SAS® version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Demographic factors and efficacy outcomes were analyzed using data from patients in the intention-to-treat population of the SETTLE study (i.e., all randomized patients). Safety was assessed for all patients exposed to the study medication. All analyses were carried out for Asian and Caucasian racial subgroups.
For demographic factors, categorical variables were reported as the number and percentage of subjects, and continuous variables were analyzed using descriptive statistics. To detect the difference between subgroups, Welch’s t test was used for continuous variables, and Fisher’s exact test was used for categorical variables.
For efficacy outcomes, a last-observation-carried-forward methodology was used to impute dropout and missing data at the last assessment point. Changes from baseline to week 24 in efficacy outcomes were compared between racial subgroups using analysis of covariance (ANCOVA) models that included baseline values, region, body weight, disease duration, Hoehn and Yahr stage, and concomitant use of nonlevodopa and antiPD medication as covariates.
For safety outcomes, a summary of TEAEs, TEAEs by preferred term, and severity of dyskinesia were reported as the number and percentage of subjects. Fisher’s exact test was used to compare differences between the two treatment arms within each racial subgroup. Differences between racial subgroups in the incidence and severity of TEAEs were compared through logistic regression using region, body weight, disease duration, Hoehn and Yahr stage, and concomitant use of nonlevodopa antiPD medication as covariates.
RESULTS
Demographic factors
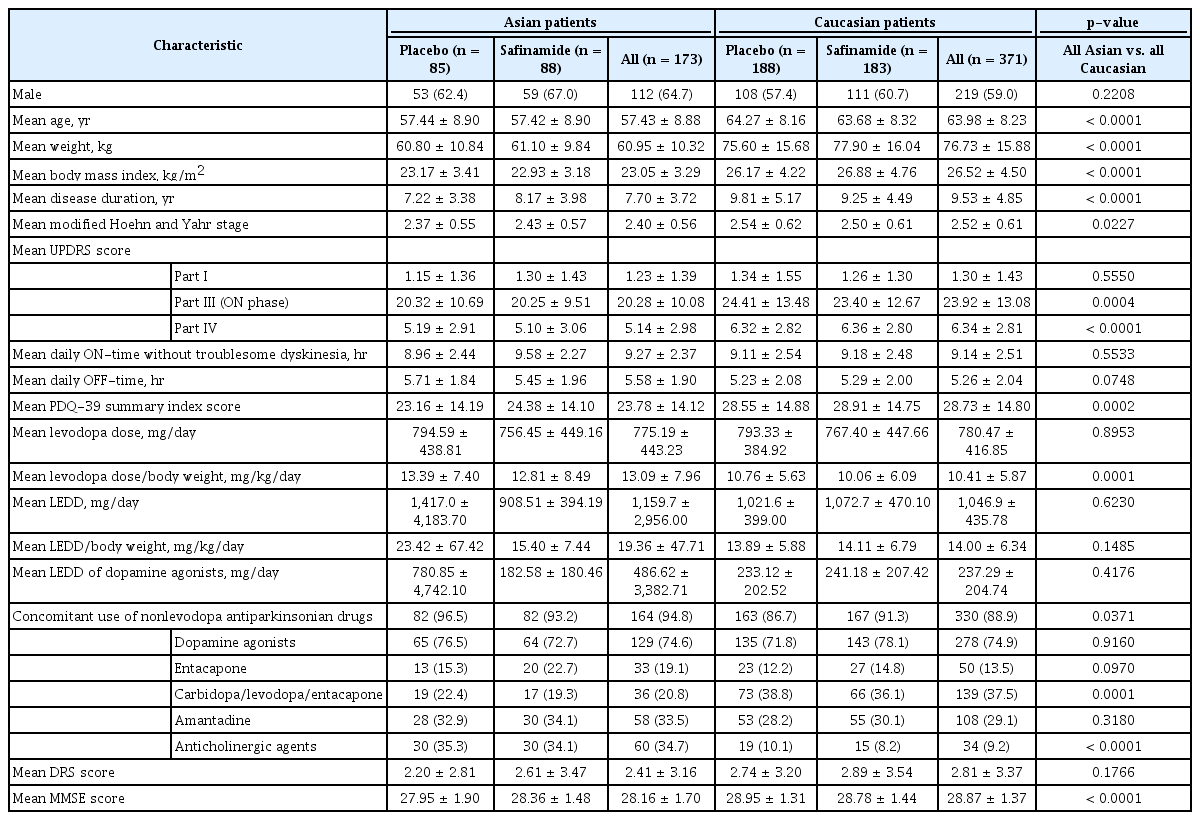
The study population (n = 544) consisted of 173 Asian and 371 Caucasian patients (Table 1). Demographic factors that were significantly different between Asian and Caucasian patients included age (57.43 vs. 63.98 years; p < 0.0001), weight (60.95 vs. 76.73 kg; p < 0.0001), body mass index (23.05 vs. 26.52 kg/m2; p < 0.0001), UPDRS Part III score (20.28 vs. 23.92; p = 0.0004), and PDQ-39 summary index score (23.78 vs. 28.73; p = 0.0002). Asian patients also had a shorter duration of disease (7.70 vs. 9.53 years; p < 0.0001) and significantly worse Mini-Mental State Examination (MMSE) scores at baseline (28.16 vs. 28.87; p < 0.0001) than Caucasian patients.
Both subgroups received similar daily doses of levodopa (775.19 vs. 780.47 mg/day); however, owing to the lower body weight of Asian patients, their levodopa dose per kilogram of body weight was significantly higher (13.09 vs. 10.41 mg/kg/day; p = 0.0001). The levodopa equivalent dose (LEDD) per kilogram of body weight was not significantly different between the two groups (19.36 [Asians] vs. 14.00 mg/kg/day [Caucasians]; p = 0.1485). At baseline, a higher proportion of Asian than Caucasian patients were receiving concomitant anticholinergic drugs (34.7% vs. 9.2%; p < 0.0001), which may reflect the usual clinical practices in Asia [22].
Efficacy outcomes
Motor fluctuations and motor symptoms
In Asian and Caucasian patients, significant improvements in the primary outcome of change in mean daily ON-time from baseline to week 24 were observed for safinamide versus placebo (Figure 1A). In Asian patients, the least-squares (LS) mean daily ON-time was 1.20 hr with placebo and 2.03 hr with safinamide (LS mean difference relative to placebo: 0.83 hr [95% confidence interval [CI]: 0.19, 1.46]; p = 0.011). For Caucasian patients, the LS mean was 0.53 hr with placebo and 1.58 hr with safinamide (LS mean difference relative to placebo: 1.05 hr [95% CI: 0.53, 1.57]; p < 0.0001).
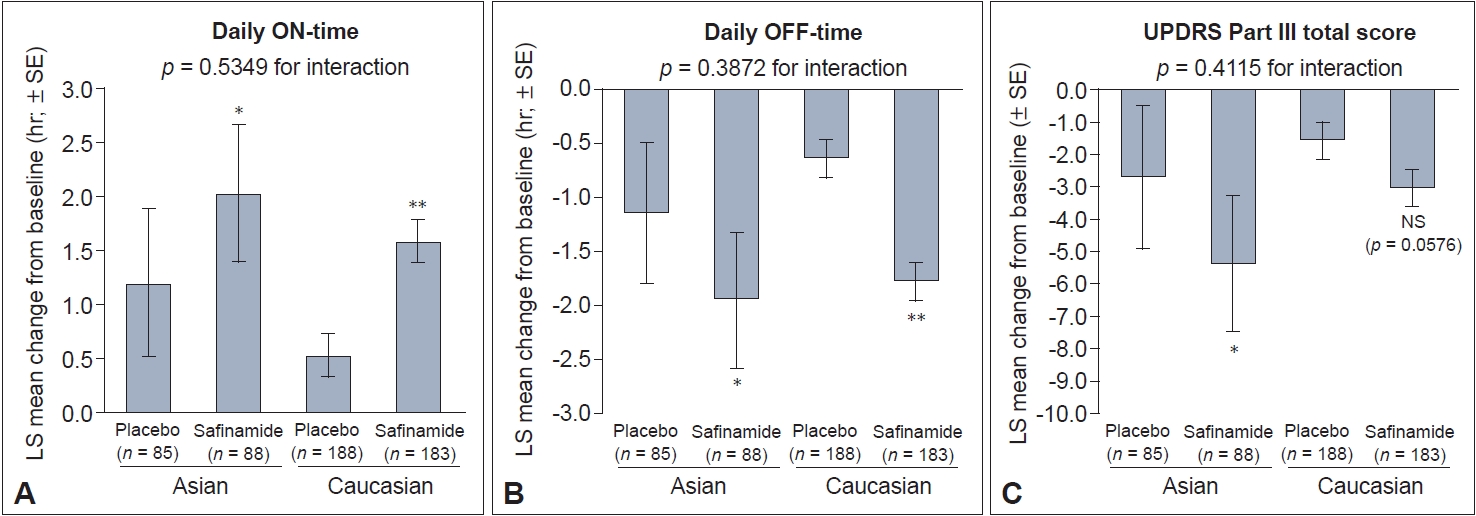
Change from baseline to week 24 in (A) daily ON-time, (B) daily OFF-time, and (C) Unified Parkinson’s Disease Rating Scale (UPDRS) Part III total score. *p < 0.05; **p < 0.01 for comparison vs. placebo. p-values for the interaction between subgroups were calculated using analysis of covariance. hr, hour; LS, least squares; NS, not significant; SE, standard error.
Both Asian and Caucasian safinamide-treated patients experienced significant reductions in daily OFF-time compared with patients who received placebo. The LS mean difference was -0.81 hr in Asians (95% CI: -1.43, -0.20; p = 0.01) and -1.14 hr in Caucasians (95% CI: -1.60, -0.68; p < 0.0001) (Figure 1B).
A significant reduction in UPDRS Part III scores was seen in Asian safinamide-treated patients compared with placebo (LS mean difference: -2.65 [95% CI: -4.71, -0.59]; p = 0.012) (Figure 1C). Although the same directional trend was seen in Caucasian safinamide-treated patients, the difference versus placebo was not statistically significant (LS mean difference: -1.44; p = 0.0576). There was no statistically significant interaction between treatment and race for any of the primary outcome measures (Figure 1).
Figure 2 shows the change from baseline to week 24 in other key secondary outcomes. No significant change in UPDRS Part II scores was observed in patients who received safinamide versus placebo in either subgroup (Figure 2A). Similar reductions in OFF-time after morning levodopa were seen for safinamide-treated patients in both subgroups (LS mean difference compared with placebo: Asians: -0.22 hr [95% CI: -0.40, -0.04]; p = 0.01; Caucasians: -0.16 hr [95% CI: -0.28, -0.04]; p = 0.01) (Figure 2B). No significant change in DRS and UPDRS Part IV scores was observed after 24 weeks of treatment for Asian or Caucasian patients, even after stratifying for patients with DRS scores > 0 and = 0 at baseline (Figure 2C, Supplementary Table 2 in the online-only Data Supplement). There was no statistically significant interaction between treatment and race for any of the key secondary outcome measures (Figure 2).
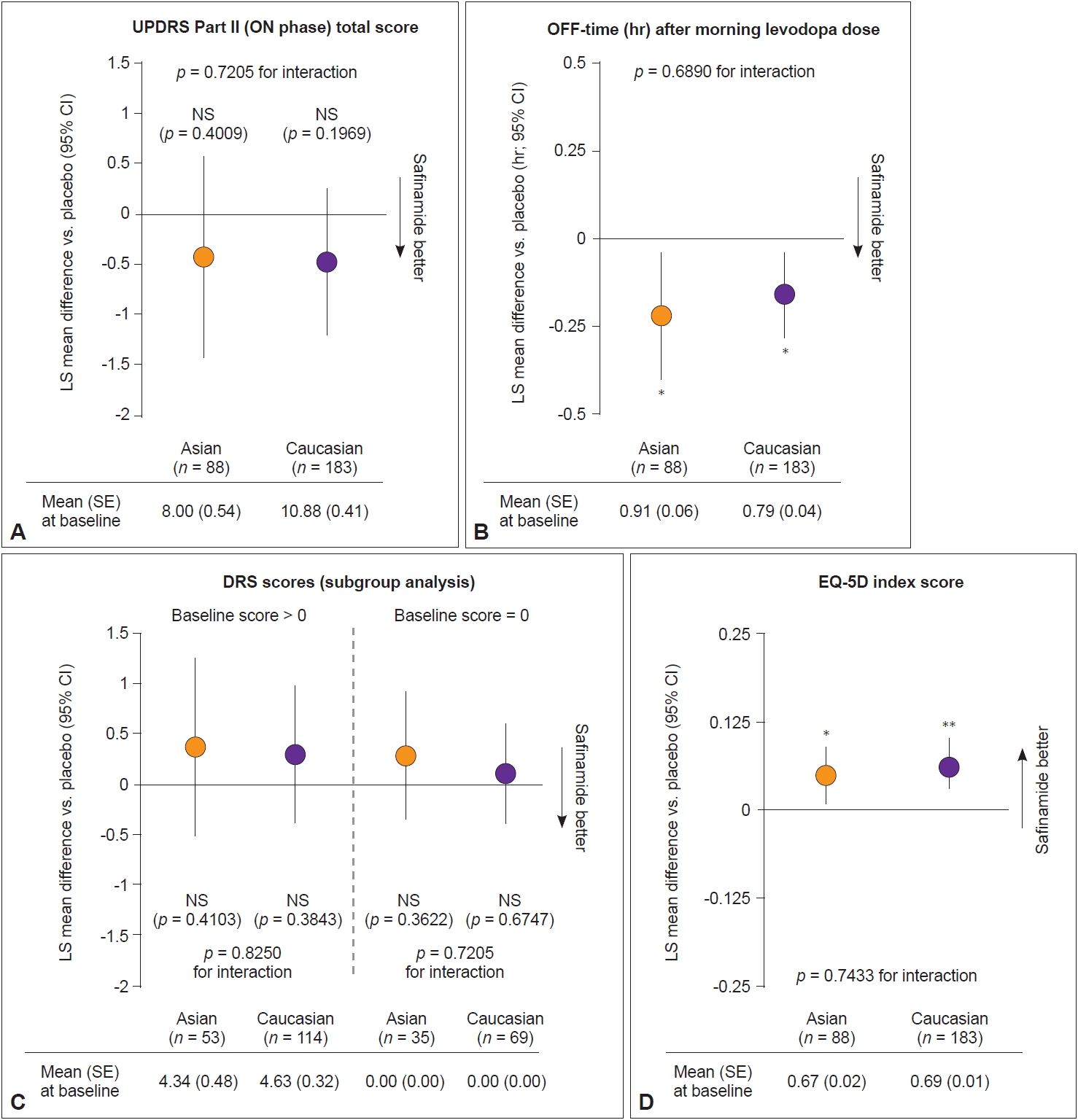
Change from baseline to week 24 in key secondary outcomes. A: Unified Parkinson’s Disease Rating Scale (UPDRS) Part II (ON phase) total score. B: OFF-time (hr) after morning levodopa dose. C: Dyskinesia rating scale (DRS) scores (subgroup analysis). D: Euro-QoL five dimensions (EQ-5D) index score. Baseline mean and standard error (SE) were calculated according to the number of patients in each subgroup: Asian patients who received safinamide (n = 88) vs. placebo (n = 85); Caucasian patients who received safinamide (n = 183) vs. placebo (n = 188). *p < 0.05; **p < 0.01 for comparison vs. placebo. p-values for the interaction between subgroups were calculated using analysis of covariance. CI, confidence interval; LS, least squares; NS, not significant.
Non-motor symptoms
No significant difference in ESS and QUIP scores was observed between the safinamide and placebo groups in Asian or Caucasian patients (Supplementary Table 2 in the online-only Data Supplement). Supplementary Table 3 (in the online-only Data Supplement) reports the change from baseline in Cogtest PD Battery subdomain scores. An improvement in spatial working memory subdomain scores was observed in Asian safinamide-treated patients (LS mean difference compared with placebo: 0.81 [95% CI: 0.03, 1.58]; p = 0.04) but not Caucasian patients (p = 0.10). No other significant difference in Cogtest subdomain scores was noted.
Quality of life
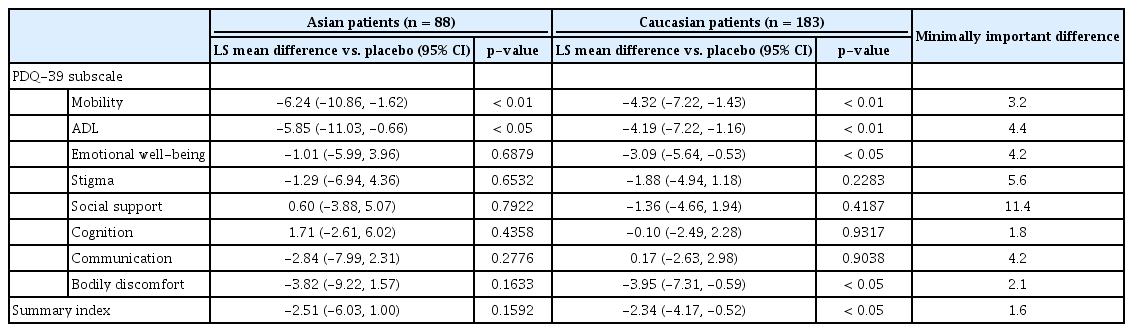
Significant improvements in EQ-5D scores were observed in safinamide-treated patients in both subgroups (Asians: 0.05, LS mean difference compared with placebo, p = 0.02; Caucasians: 0.06, LS mean difference compared with placebo, p = 0.001) (Figure 2D, Supplementary Table 2 in the online-only Data Supplement). A significant improvement in PDQ-39 summary index scores was seen in Caucasian safinamide- versus placebotreated patients (p < 0.05). The improvement was not significant in Asian patients (p = 0.16), although the effect size of improvement for safinamide-treated patients was similar (Table 2, Supplementary Table 4 in the online-only Data Supplement). Significant improvements in scores for the PDQ-39 subdomains of mobility and ADL were seen in both Asian and Caucasian safinamide-treated patients relative to placebo (Table 2, Supplementary Table 4 in the online-only Data Supplement).
Safety outcomes
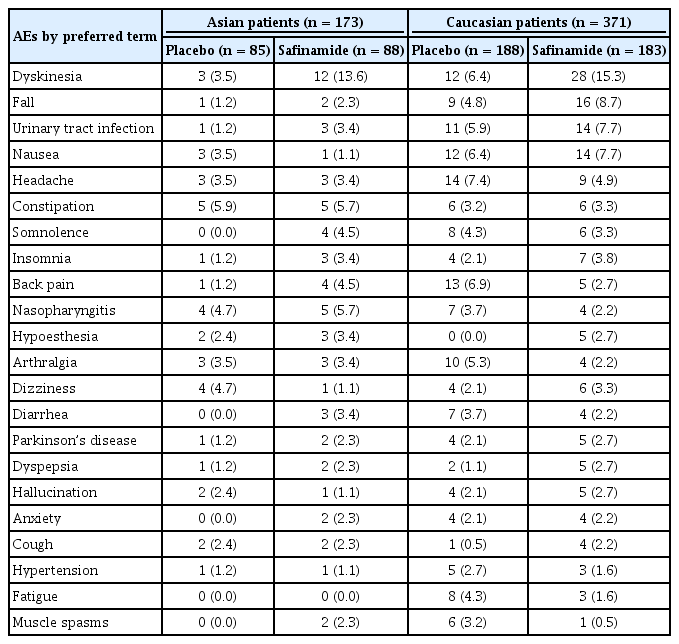
The incidence of TEAEs was similar for safinamide- and placebo-treated patients in each subgroup (Tables 3 and 4). Most safinamide-treated patients experienced mild TEAEs (Asians: 55.7%; Caucasians: 61.7%), and fewer Asian than Caucasian safinamide-treated patients had moderate TEAEs (21.6% vs. 36.1%). There was no statistically significant interaction between treatment and race for the incidence or severity of TEAEs (Table 3).
None of the Asian safinamide-treated patients experienced TEAEs leading to study discontinuation, while 12 Caucasian patients (6.6%) discontinued the study because of TEAEs. Dyskinesia was the most common AE in both subgroups (Asians: 13.6%, p = 0.0281; Caucasians: 15.3%, p = 0.0070; p [interaction] = 0.6947). For safinamide-treated Asian patients, the most frequent AEs (> 5%) by incidence were dyskinesia, constipation, and nasopharyngitis (5.7% each). The most common AEs in Caucasian safinamide-treated patients were falls (8.7%), urinary tract infections, and nausea (7.7% each). Among Caucasian safinamide-treated patients, 2.2% experienced orthostatic hypotension, which was not reported in Asian safinamide-treated patients.
Further analysis of the severity of dyskinesia showed that most safinamide-treated Asian patients experienced mild dyskinesia (8.0%), while most Caucasian patients experienced moderate dyskinesia (9.3%). There was no statistically significant interaction between treatment and race for the severity of dyskinesia (p = 0.7166).
DISCUSSION
In this post hoc analysis of data from the SETTLE study, we found that safinamide as an adjunct to levodopa is effective in improving daily ON-time and motor functions in Asian and Caucasian patients with PD and is well tolerated by both patient groups. To our knowledge, this is the first analysis exploring the efficacy and safety of safinamide treatment for Asian patients with PD from a large, international, multicenter trial. Although it is a post hoc analysis with inherent limitations, it is nevertheless an important addition to the literature, providing new evidence on the efficacy and safety of PD treatments for a population that remains largely understudied, as most clinical studies in PD have been conducted in Western populations and data on treatment outcomes specific to Asian patients are limited [5,21].
Efficacy profiles
Safinamide was effective in reducing the impact of wearing-off symptoms for Asian and Caucasian patients, and significant improvements in change from baseline to week 24 in the primary endpoint of mean daily ON-time were observed in both subgroups. Relative to placebo, safinamide-treated patients from both subgroups also experienced a reduction in daily OFF-time from baseline by approximately 1 hr, with the magnitude of reduction being similar to that in a Phase II/III Japanese trial of safinamide (LS mean difference compared with placebo: 1.25 hr in 50 mg/day group, 1.72 hr in 100 mg/day group) [11]. Reductions in morning OFF-time after levodopa were also seen in both Asian and Caucasian patients.
Overall, motor-function benefits with safinamide were observed, as UPDRS Part III scores improved significantly (-1.82 relative to placebo) [12]. In our subgroup analysis, significant improvements were observed among Asian patients, with UPDRS Part III scores improving by 2.65 points relative to placebo (-5.38 from baseline), but not among Caucasian patients (-3.03 from baseline). Based on clinically important differences reported for the UPDRS motor score, these are considered clinically meaningful changes in response to therapeutic interventions in PD for both subgroups (minimal change: -2.5; moderate change: -5.2; large change: -10.8) [23]. Changes to individual motor symptoms in UPDRS Part III were not analyzed in the present study.
While this study presents important data on the efficacy and safety of safinamide for Asian patients with PD, key differences were present in the demographic and clinical profiles of Asian and Caucasian patients that must be considered when comparing specific patient outcomes. This was a limitation of the study’s post hoc nature. For example, Asian patients were significantly younger than Caucasian patients (approximately 7 years younger), had a shorter duration of disease (approximately 2 years shorter), and were more likely to be receiving amantadine and anticholinergic treatment. An important significant difference between the groups was the lower average body weight of Asian patients with PD (approximately 15 kg lower). While Asian and Caucasian patients received the same dose of safinamide and comparable doses of levodopa (approximately 770 mg/day), the significantly lower body weight of Asian patients resulted in a higher average levodopa dose per kilogram of body weight (approximately 2.5 mg/kg/day higher). As body weight affects the pharmacokinetic properties of levodopa and is related to its blood concentrations, Asian patients in the SETTLE study were likely to have higher levodopa blood concentrations than Caucasian patients [24]. In testing for interactions between treatment and racial subgroup for efficacy outcomes, we included baseline values, body weight, disease duration, Hoehn and Yahr stage, and concomitant use of nonlevodopa anti-PD medication as covariates in the analysis. When we controlled for these factors, there were no statistically significant interactions between treatment and race for any of the outcomes analyzed.
Importantly, safinamide treatment did not worsen DRS scores in Asian or Caucasian patients, even in those with preexisting dyskinesia at study enrollment. Although it is common clinical practice to reduce or discontinue MAO-B inhibitors in patients with troublesome dyskinesia, the current analysis suggests that safinamide is unlikely to exacerbate mild dyskinesia [25]. Early treatment with amantadine can reduce the onset of levodopa-induced dyskinesia, and given safinamide’s anti-glutamatergic action, it is possible that safinamide could act in a similar manner to amantadine [8,26]. This effect of safinamide is consistent with a post hoc analysis of a Japanese Phase III trial [27]. Together, these findings may help to inform clinical-practice decisions for doctors in Asia.
Non-motor symptoms in PD can have an even greater impact on patients’ QoL than motor symptoms and are highly prevalent among patients with PD [19], including across different ethnic groups [28,29]. Based on Cogtest PD Battery scores, this analysis found that safinamide was not associated with worsening of cognitive function. This is consistent with another study in Europe, which did not find any reduction in cognitive function for safinamide-treated patients [30].
Interestingly, the current analysis also found an improvement in spatial working memory scores for Asian patients. This is consistent with an exploratory analysis of patients with fluctuating PD, in which patients had significant improvements in Frontal Assessment Battery and Stroop Word Color Test scores after 12 weeks of safinamide treatment [31]. Importantly, compared with Caucasian patients, Asian patients in the SETTLE study received a higher baseline level of concomitant anticholinergic agents, which are known to adversely affect cognitive function [32]. Increased use of anticholinergic drugs among Asian patients is also consistent with observed clinical practices and clinician preferences in the region [22,33]. One possible explanation for our findings is that safinamide may mitigate some of the effects of anticholinergic drugs on working memory. However, it is difficult to interpret these data conclusively, as the SETTLE study excluded patients with cognitive dysfunction. Consequently, further studies are needed to fully determine the effects of safinamide on cognition.
Asian patients in the SETTLE study received high doses of levodopa and had numerically higher QUIP scores at baseline than Caucasians (Supplementary Table 2 in the online-only Data Supplement). The addition of safinamide did not worsen QUIP scores in either subgroup, suggesting that the risk of impulse-control disorders with safinamide may be low. However, patients with dementia, cognitive dysfunction (MMSE < 22), and depression (GRID-Hamilton Depression Rating Scale [GRID-HAMD] > 17) were excluded from the trial [12]. The Non-motor Symptom Scale was also not used in this study, so further studies are needed to comprehensively characterize the effects of safinamide on non-motor symptoms in Asian patients.
Safinamide significantly improved EQ-5D scores in Asian and Caucasian patients and led to improvements in QoL based on this well-validated and sensitive non-PD-specific instrument [34]. Significant improvements in the PDQ-39 summary index were seen in Caucasian patients, with a nonsignificant trend toward improvement in Asian patients. Within subdomains of the PDQ39 index, Asian and Caucasian patients showed significant improvements in mobility and ADL. Additionally, a significant improvement in bodily discomfort was seen in Caucasian safinamide-treated patients relative to placebo-treated patients. Although these results (PDQ-39 summary index and bodily discomfort) were not significant in Asian patients, the effect size was similar, and the LS mean differences in scores are considered clinically relevant, as they exceed the minimal clinically important difference for PDQ-39 [35].
Safety profiles
No major differences in the incidence of TEAEs were observed between Asian and Caucasian safinamide-treated patients. Aside from dyskinesia, which was more common in safinamide-treated patients, the incidence of AEs was similar for safinamide-and placebo-treated patients, although constipation was more frequent in Asian patients, consistent with previous studies that found a high incidence of constipation and gastrointestinal disturbance in Asian patients compared with Western populations [36].
All Asian patients in the SETTLE study tolerated the 100 mg/day dose of safinamide, and none discontinued the trial. Most safinamide-treated Asian patients experienced mild dyskinesia, while Caucasian patients experienced moderate dyskinesia. Unlike Caucasian patients, no Asian safinamide-treated patients experienced orthostatic hypotension. Despite the low body weight and potentially high blood concentrations of levodopa and safinamide in Asian patients, safinamide was well tolerated.
Response to treatment and disease progression may vary between ethnically diverse patients for many reasons, including genetic factors [18,20,37]. However, consideration of how genetic factors may contribute to the observed differences in response to safinamide is beyond the scope of the current analysis, as the SETTLE study was neither designed nor powered to determine factors that may drive differences in treatment response between the ethnic groups included. Therefore, based on the data available, we cannot postulate why Asians and Caucasians may respond differently, and further studies are needed to provide a deeper understanding of the effect of genetic and other factors on the safinamide response.
Another study limitation is that, as this was a post hoc analysis, the statistical analysis plan was neither prespecified nor powered to detect differences in treatment effect by subgroup. The study’s post hoc nature also resulted in imbalances in baseline demographic factors between the two subgroups, which, as noted above, may have influenced study outcomes and made interpretation of some of the data difficult. Potential confounding factors exist, such as age, body weight, duration of disease, disease severity, and concomitant medications. Nonetheless, previous post hoc analyses of the data included in this study have suggested that certain baseline characteristics that differed between Asian and Caucasian patients did not impact the efficacy of safinamide. For example, a previous subgroup analysis from a Japanese study found that patients receiving 100 mg/day safinamide had significant improvements in mean daily ON-time, OFF-time, and UPDRS Part III score relative to placebo regardless of baseline UPDRS Part III score [38]. Thus, the difference in baseline UPDRS Part III score between groups in the present study is not expected to lead to discrepancies in safinamide efficacy between Asian and Caucasian patients. Furthermore, there were no statistically significant interactions between treatment and race for any of the outcomes analyzed when controlling for potential confounding variables.
Finally, the findings of our study are also limited in their generalizability: the heterogeneity of ethnicities across Asia precludes generalizing these results for all patients in the region, although patients from several countries across Asia were included in this study. This may affect interpretation of the results, and further studies are needed for a more detailed understanding of the effect of safinamide on motor and non-motor symptoms.
Conclusion
In this post hoc analysis, safinamide significantly improved daily ON-time without troublesome dyskinesia and reduced wearing-off symptoms in both Asian and Caucasian patients with PD and motor fluctuations.
Safinamide was well tolerated in both subgroups, with Asian patients able to tolerate the 100 mg/day dose. Safinamide treatment did not worsen the DRS score in either subgroup, regardless of the presence of dyskinesia at baseline. Additionally, Asian patients in this study were exposed to a higher dose of levodopa per kilogram of body weight.
While our study represents an important first in highlighting the effects of safinamide in under researched Asian populations, future studies to assess the real-world effectiveness and safety of safinamide in Asia will be important for informing clinical-practice decisions.
Supplementary Material
The online-only Data Supplement is available with this article at https://doi.org/10.14802/jmd.22196.
SETTLE study enrollment by region and country Regio
Changes from baseline to week 24 in secondary outcomes Outcome measure
Changes from baseline to week 24 in Cogtest PD battery subdomain scores
Changes from baseline to week 24 in PDQ-39 subdomain scores
Notes
Conflicts of Interest
Employees of Eisai Co. Ltd. were involved in the study design; the collection, analysis, and interpretation of data; and the decision to submit the article for publication. Roongroj Bhidayasiri, Shey Lin Wu, Jin Whan Cho received support for the present manuscript including the provision of study materials and medical writing support from Eisai Co. Ltd., as well as consulting fees from Eisai Co. Ltd.
Funding Statement
This work was sponsored by Eisai Co. Ltd. The clinical trial was sponsored by Newron Pharmaceuticals and Merck Serono.
Author contributions
Conceptualization: Roongroj Bhidayasiri, Takayuki Ishida. Data curation: Ippei Suzuki. Formal analysis: Ippei Suzuki. Funding acquisition: Takayuki Ishida. Investigation: Takayuki Ishida, Takanori Kamei, Ryan Edbert Husni, Ippei Suzuki. Methodology: Takayuki Ishida, Takanori Kamei, Ryan Edbert Husni, Ippei Suzuki. Project administration: Takayuki Ishida. Supervision: Roongroj Bhidayasiri, Takayuki Ishida. Validation: Ippei Suzuki. Visualization: Takayuki Ishida, Takanori Kamei. Writing—original draft: Takayuki Ishida. Writing—review & editing: Roongroj Bhidayasiri, Takanori Kamei, Ryan Edbert Husni, Ippei Suzuki, Shey Lin Wu, Jin Whan Cho.
Acknowledgements
The authors would like to thank the patients who participated in this trial and their families, as well as the staff at all investigational sites. We would also like to thank Dr Shen Yang Lim from the University of Malaya for his valuable insights and contribution to the analysis and Michinori Koebisu and Yuki Kogo of Eisai Co. Ltd. for their contribution in reviewing all drafts. We thank Malvika Katarya and Liam Wilson of AMICULUM Asia for providing medical writing support, which was funded by Eisai Co. Ltd., and carried out in compliance with the Good Publication Practice 2022 guidelines.