Gastrointestinal Dysfunction in Parkinson’s Disease: Neuro-Gastroenterology Perspectives on a Multifaceted Problem
Article information
Abstract
Patients with Parkinson’s disease (PD) face a multitude of gastrointestinal (GI) symptoms, including nausea, bloating, reduced bowel movements, and difficulties with defecation. These symptoms are common and may accumulate during the course of PD but are often under-recognized and challenging to manage. Objective testing can be burdensome to patients and does not correlate well with symptoms. Effective treatment options are limited. Evidence is often based on studies in the general population, and specific evidence in PD is scarce. Upper GI dysfunction may also interfere with the pharmacological treatment of PD motor symptoms, which poses significant management challenges. Several new less invasive assessment tools and novel treatment options have emerged in recent years. The current review provides an overview and a practical approach to recognizing and diagnosing common upper and lower GI problems in PD, e.g., dyspepsia, gastroparesis, small bowel dysfunction, chronic constipation, and defecatory dysfunction. Management aspects are discussed based on the latest evidence from the PD and general populations, with insights for future research pertaining to GI dysfunction in PD.
INTRODUCTION
Patients with Parkinson’s disease (PD) suffer from a wide spectrum of motor and non-motor symptoms, including gastrointestinal (GI) dysfunction. Notably, GI dysfunction has been recognized as a feature of PD since its first detailed description by James Parkinson in his 1,817 treatise, where he vividly described these bothersome symptoms: “… the bowels, which had been all along torpid, now, in most cases, demand stimulating medicines of very considerable power: the expulsion of feces from the rectum sometimes requiring mechanical aid.” [1] GI symptoms may occur early in PD or as prodromal symptoms that predate a PD diagnosis by years or decades [2,3]. GI problems also tend to accumulate and worsen during the course of PD [3], with around 60%–80% of patients eventually experiencing GI issues [4]. These symptoms are often challenging to manage, significantly impair quality of life, and lead to potential complications, such as weight loss, malnutrition, and frailty in PD patients [5].
Similar to many neurological disorders, the GI symptoms in PD are primarily due to abnormalities in motor (rather than secretory or sensory) functions of the GI tract that lead to impaired motility [6]. The pathophysiology of GI dysfunction in PD is not well understood and is likely multifactorial. Potential mechanisms include damage and dysfunction of the intrinsic and extrinsic nervous system of the gut (e.g., the enteric nervous system (ENS), vagus nerve, and dorsal motor nucleus of the vagus nerve), alterations in the gut microbiome, gut barrier dysfunction, inflammation, dysregulation of neurogastrointestinal hormones and signaling (e.g., cholecystokinin and ghrelin), and adverse effects of antiparkinsonian medications [1,7,8]. GI dysfunction in PD may be partially related to the presence of aggregated alpha-synuclein and neuronal loss (i.e., the neuropathological hallmarks of PD) in the ENS. However, correlations between these pathological changes and clinical symptoms have not been convincingly demonstrated, and concrete evidence of neuronal loss within the ENS is lacking [9].
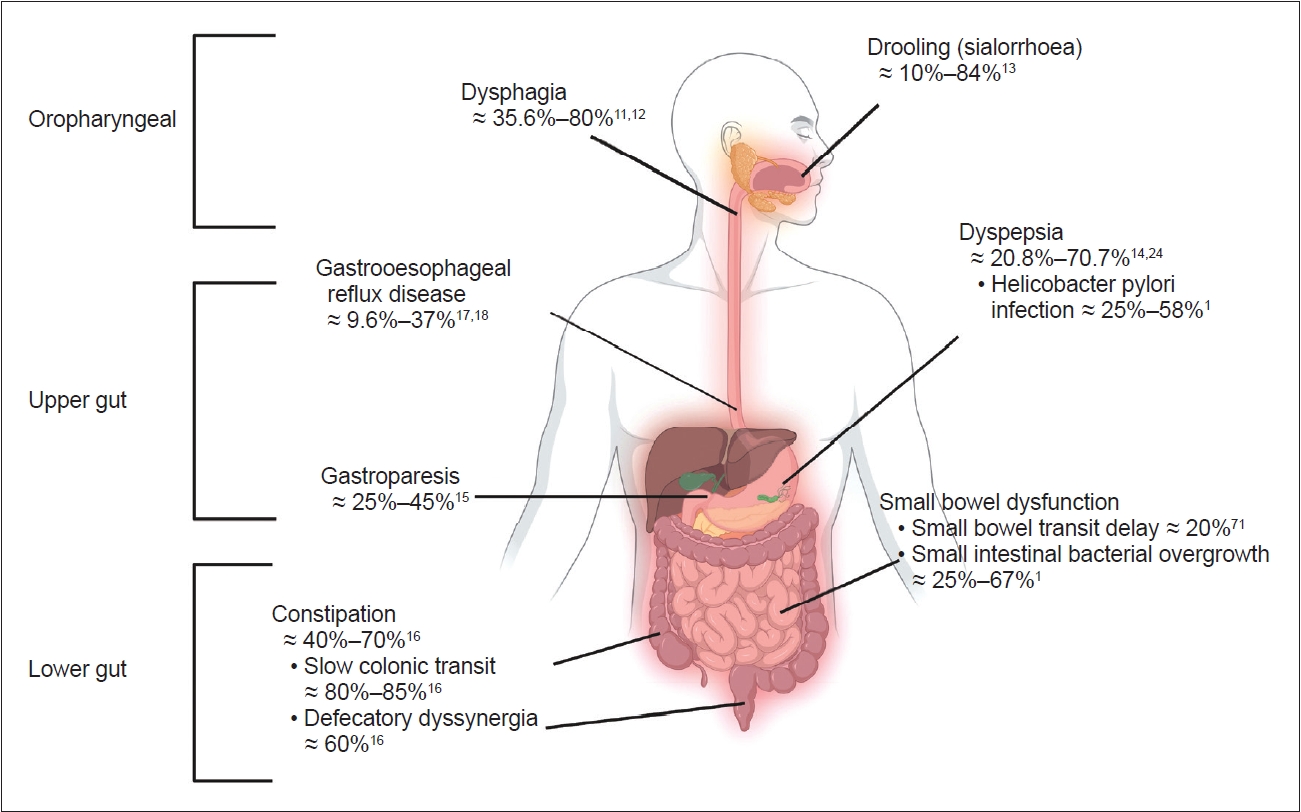
The entire GI tract is affected in PD [7,10] and leads to a wide variety of issues, including oropharyngeal and upper-mid gut (stomach and small bowel) and lower gut (large bowel) problems (Figure 1) [1,11-18]. Oropharyngeal problems, such as sialorrhea and dysphagia, are prevalent in advanced PD and were recently reviewed elsewhere [10,19]. Gastric and bowel dysfunction are prevalent in early and later stages of PD, and manifest as heterogeneous upper and lower GI symptoms. Although most GI disorders, such as dyspepsia, gastroparesis, constipation, and anorectal dysfunction, are traditionally taught as separate entities, emerging evidence suggests overlaps between these conditions. GI symptoms in PD correlate poorly with objective assessments of GI tract function. Taken together, the complexity of GI symptoms in PD likely indicates multifaceted pathophysiological processes in the GI tract, which may also interfere with the pharmacological treatment of PD motor symptoms.
This review dissects upper and lower GI symptoms in PD and discusses practical approaches to several GI disorders in PD, including dyspepsia, gastroparesis, small bowel dysfunction, chronic constipation, and defecatory dysfunction. We combined perspectives and expertise from neurology and gastroenterology spheres to present up-to-date, evidence-based strategies for the management of PD patients with GI dysfunction.
UPPER GASTROINTESTINAL ISSUES IN PARKINSON’S DISEASE
Clinical Vignette: A 58-year-old woman with PD diagnosed four years previously complained of increasingly troublesome bloating, nausea, and occasional epigastric discomfort over the past six months. She vomited several times in the past few weeks. Her medications included selegiline (5 mg b.i.d.), pramipexole (0.375 mg b.i.d.), and recently added levodopa-benserazide (50/12.5 mg b.i.d.).
The term ‘dyspepsia’ refers to a symptom complex that originates from the gastroduodenal region [20]. These symptoms include discomfort or pain in the upper abdomen, epigastric fullness, bloating, early satiety, nausea, and/or vomiting [21], which are sometimes broadly termed by patients as ‘indigestion’. These symptoms in PD may be due to a variety of upper GI problems, including dyspepsia, gastroparesis, small intestinal bacterial overgrowth (SIBO), and GI dopaminergic side effects of PD medications, as discussed below.
Dyspepsia and Helicobacter pylori infection
Dyspepsia may be due to organic or structural causes, such as gastroesophageal reflux disease (GERD), peptic ulcer disease (and Helicobacter pylori [H. pylori] infection), GI malignancy, and biliary and pancreatic disorders [21]. Notably, approximately 80% of adults with dyspepsia in the general population have no structural explanation for their symptoms and are termed as having functional dyspepsia (FD) [21]. Diagnosed using the Rome IV criteria, functional dyspepsia is divided into postprandial distress syndrome (FD-PDS), which is characterized by bothersome after meal-related symptoms, and epigastric pain syndrome (FD-EPS), which may overlap [20,21]. Dyspepsia is common and affects up to 20% of the world’s population at any one point in time, and is sometimes very challenging to manage [22,23].
Despite its considerable burden, the literature on dyspepsia in PD is surprisingly sparse. One study found that approximately 20.8% of early PD patients (disease duration < 3.5 years) experienced early satiety [24]. Researchers documented using the Leeds Dyspepsia Questionnaire that PD patients had more frequent/severe dyspeptic symptoms than non-PD controls [25], and excessive fullness/bloating and upper abdominal pain were reported as the most troublesome dyspeptic symptoms in two studies [25,26]. Moreover, postprandial dyspeptic symptoms (e.g., indigestion and excessive fullness/bloating) correlated with poorer quality of life in PD patients [27]. Approximately 10%–37% of PD patients have GERD symptoms (e.g., heartburn and reflux/regurgitation [17,28,29]), which frequently overlap with dyspepsia. Notably, several upper GI conditions (e.g., dyspepsia, GERD, and H. pylori infection) were associated with an increased risk of developing PD in large epidemiological studies [30-33].
Patients with dyspepsia and ‘red flag’ features (e.g., overt GI bleeding, dysphagia/odynophagia, persistent vomiting, unintentional/rapid weight loss, iron deficiency anemia, and family history of GI malignancy) should have upper endoscopy, laboratory tests, or imaging to exclude serious organic causes [34]. Empirical therapy should be considered in patients without red flag features. Expert guidelines from Europe and Asia recommend proton pump inhibitors (PPIs) and prokinetics as initial therapy for FD-EPS and PDS subtypes, respectively [35,36]. Prokinetics may provide additional advantages in PD patients who may have concomitant gastroparesis (discussed further below). In contrast, North American guidelines recommend PPI as the initial therapy for FD regardless of the subtype [37]. A recent network meta-analysis ranked standard-dose PPIs as the best agent to achieve symptom resolution in FD (relative risk [RR]: 0.86 vs. placebo), and domperidone was not efficacious (RR: 1.20 vs. placebo) [23].
If first-line medications for FD are not successful, neuromodulators, e.g., tricyclic antidepressants (TCAs), are recommended as second-line therapy [20,23]. Notably, several TCAs (nortriptyline and desipramine) were designated ‘likely efficacious’ in the treatment of PD depression by the International Parkinson and Movement Disorder Society (MDS) Evidence-based Medicine (EBM) Committee [38]. Taken together, these agents are reasonable treatment options for PD patients who have concomitant depression and bothersome dyspepsia but should be used with caution in patients with constipation, urinary retention, angle-closure glaucoma, cardiovascular disorders, and cognitive dysfunction [38]. Several small and short-term trials also demonstrated the efficacy of antipsychotics (sulpiride or levosulpiride) with fairly large effect sizes in treating FD [23]. However, these agents block dopamine centrally and may worsen parkinsonism and should be avoided in PD patients. The effects of other antipsychotics recommended in the treatment of PD psychosis (clozapine, quetiapine, and pimavanserin) [38] on FD have not been systematically examined. Psychotherapy (e.g., psychodynamic interpersonal therapy, cognitive behavioral therapy, or hypnotherapy) may be considered, especially in cases of intractable FD or when the patients have additional psychological comorbidities. However, the availability of these ancillary services may be lacking in many parts of the world [39,40]. Specific clinical trials for dyspepsia in PD are relatively lacking. Treatment with the plant-based prokinetic DA-9701 for 12 weeks improved dyspepsia severity scores in a double-blind randomized placebo-controlled trial (DB-RPCT) (n = 144), and no serious adverse events were reported [41].
H. pylori infection, which has been causatively linked to dyspepsia, peptic ulcer disease, and gastric carcinoma, is a major public health issue worldwide, especially in Asia, Africa, and Latin America [42]. Guidelines recommend that patients with dyspepsia (with or without red flag symptoms) should undergo H. pylori testing, which is easily performed using a non-invasive [13] C urea breath test or a stool antigen test [34]. The prevalence of H. pylori infection ranges between 25%–58% in PD, which is comparable to local non-PD populations [1]. H. pylori eradication therapy was superior to standard therapy/placebo for the treatment of dyspepsia in a recent large meta-analysis [43], but a DB-RPCT of H. pylori eradication (n = 80) found no benefit in reducing dyspepsia severity in a PD population [44].
Gastroparesis
Gastroparesis is characterized by delayed gastric emptying (GE) in the absence of a mechanical outlet obstruction of the stomach, which results in the cardinal symptoms of early satiety, postprandial fullness, nausea, vomiting, belching, and bloating [45]. There is significant symptom overlap between gastroparesis and FD, especially the PDS subtype [46]. Notably, recurrent or persistent vomiting is not typical of dyspepsia, and an alternative diagnosis, such as gastroparesis, should be considered. 21 The prevalence of gastroparesis is 13.8 per 100,000 persons in the general population [47]. Diabetes mellitus is an important etiology and accounts for approximately one-third of all cases of gastroparesis [45,48]. However, a significant proportion (around 40%) of patients have idiopathic gastroparesis (i.e., without identifiable etiology) [48].
The true prevalence of gastroparesis in PD is uncertain. Most studies involved only small sample sizes and reported a wide range of GE times among subjects, which indicated high interindividual variability [15,49]. Meta-analyses also revealed substantial heterogeneity between studies due to methodological differences, including inclusion criteria, diagnostic methods, criteria (e.g., cut-off for normal values), and protocol (e.g., solid vs. liquid meal composition and timing of assessment in relation to PD medication intake) [49,50]. GE is delayed in PD patients vs. controls, and a more robust effect size was found in breath test studies compared to gastric scintigraphy studies [49,50]. Notably, some studies also found that a subset of PD patients had accelerated GE [49,51]. Gastroparesis may occur at any stage of PD. Although earlier studies in individuals without PD demonstrated that levodopa treatment delayed GE, studies of patients with PD did not consistently support this result [15,49,52]. The occurrence of gastroparesis in untreated PD patients [49,53] also indicates that the disease itself, rather than PD medications, may be primarily responsible for delayed GE.
Symptoms of gastroparesis correlate poorly with GI motility tests. Accelerated and delayed GE are observed in patients with similar symptoms. 54 The positive predictive value for clinical suspicion was only 29% in a cohort of 572 patients with presumed gastroparesis who underwent gastric scintigraphy [55]. Therefore, the International Working Group for Disorders of Gastrointestinal Motility and Function recommended an objective assessment of GE time in patients with persistent troublesome symptoms or poor response to therapy [54]. The gold standard GE test, gastric scintigraphy, requires substantial technical expertise and protocol optimization. Because the results are dependent on the meal content, a standardized protocol using a low-fat egg-white meal is recommended, and retention > 60% at 2 hours and > 10% at 4 hours are the proposed cut-offs for delayed GE [54]. As an alternative, the 13 C-breath test is simpler to perform but it is less reliable because many confounders affect its results, including physical activity, malabsorption, bacterial overgrowth, pancreatic exocrine insufficiency, and lung or liver disease [49,54]. Both tests are time consuming (requiring at least four hours to complete) and may be demanding for PD patients, especially patients who are frail or significantly disabled. The wireless motility capsule (WMC) recently emerged as a useful non-invasive test and has shown overall fair agreement with gastric scintigraphy results (50%–75%) with an additional advantage of detecting extra-gastric abnormalities [45,54,56]. Although the WMC is now FDA-approved for the evaluation of GE, other international guidelines have not endorsed this device as a valid test, and further validation studies in patients with gastroparesis are required [46,57].
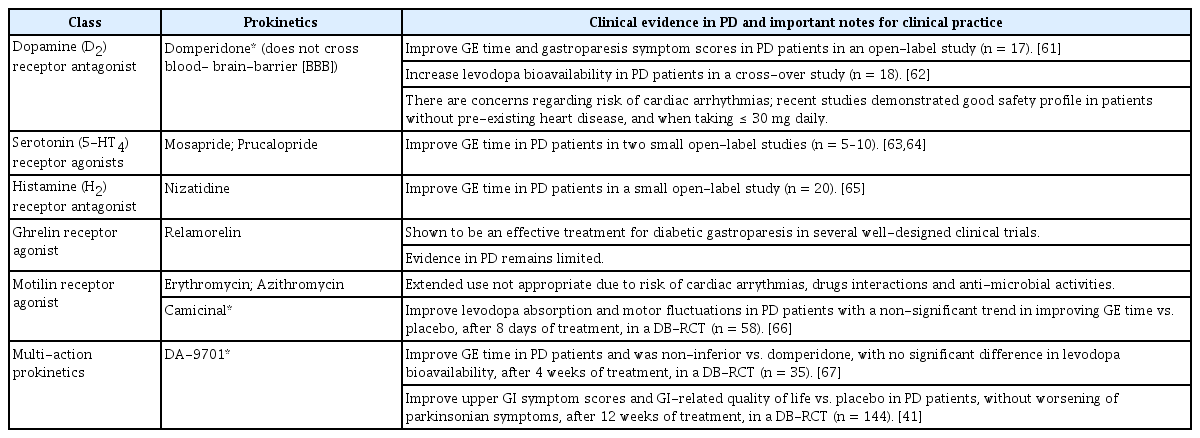
Therapeutic options for gastroparesis are somewhat limited. Dietary adjustments and nutritional support are recommended as first-line management. Patients should be advised to eat small, frequent meals and avoid high-fat diets [45]. Caloric liquids, such as soups, are encouraged [58]. Notably, a small particle size diet (e.g., food that has been mashed or pureed) improved upper GI symptoms and GE in diabetic patients with gastroparesis [59,60]. Prokinetics are the primary pharmacotherapy for gastroparesis. However, specific evidence in PD is relatively lacking (Table 1) [4,15,41,61-67]. Domperidone is a peripheral dopamine D2 receptor antagonist that is relatively impermeable to the blood-brain barrier, and it is a commonly used prokinetic agent. Domperidone improved GE time, gastroparesis symptoms and levodopa bioavailability in two small studies in PD [61,62]. Although concerns have been raised about potential cardiotoxicity (prolongation of the QT interval and ventricular arrhythmias), other studies suggested an acceptable safety profile in patients without pre-existing heart disease and at doses ≤ 30 mg daily [15,58]. In contrast, metoclopramide crosses the blood-brain barrier and is contraindicated in PD because it worsens parkinsonism [58]. Although serotonin receptor (5-HT 4) agonists are an effective option for gastroparesis in the general population [68], the potential benefits of prucalopride and mosapride in PD were only demonstrated in small open-label studies [63,64]. Cisapride and tegaserod have been withdrawn due to cardiotoxity [58]. The histamine receptor agonist nizatidine ameliorated gastroparesis in a small open-label study [65]. The motilin receptor agonist camicinal improved levodopa bioavailability and motor fluctuations in PD in a DB-RPCT (n = 58) and exhibited a non-significant trend in improving GE time compared to placebo. 66 DA-701 improved upper GI symptoms and dyspepsia (as described above) [41], and it improved GE time vs. placebo in a separate DB-RPCT (n = 37) [67]. DA-9701 exerted multiple actions on GI tract receptors (5-HT4 [+], 5-HT1A [+], α2 [+], and D2 [-]) in vivo, with negligible central anti-dopaminergic activities [67]. Although gastric electric stimulation and mechanical pyloric intervention have produced variable success for medically refractory gastroparesis, the level of evidence for both interventions is low. The results from small open-label studies on botulinum toxin injection into the pyloric sphincter in PD are conflicting [69]. A small randomized controlled trial (n = 19) did not find significant benefit from transcutaneous vagal nerve stimulation in the treatment of gastroparesis in PD [70]. Enteral feeding via a jejunal tube or parenteral nutrition may be considered as a last resort for refractory gastroparesis [57].
Small bowel dysfunction and small intestinal bacterial overgrowth
The literature on small bowel dysfunction in the general population and PD is limited, which is likely a consequence of its relative inaccessibility. Twenty percent of PD patients had small bowel transit delay in a study using WMC (n = 65) [71]. Another study using a 3D-transit magnetic tracking system found significantly longer small bowel transit in PD patients (n = 22; ≤ 400 minutes) compared to controls (n = 15; ≤ 295 minutes) [72]. Most studies on small bowel dysfunction in PD investigated the presence of SIBO, which is an acquired disorder defined by the presence of excessive numbers of bacteria in the small intestine, typically coliform bacteria that generally reside in the colon [73,74]. Abdominal bloating is the most common presenting symptom of SIBO, and abdominal pain, diarrhea, and flatulence are other common symptoms [73,74]. Several conditions are associated with an increased risk of SIBO, including gut dysmotility, which is common in PD, and gastric hypochlorhydria. Therefore, H. pylori infection is a risk factor for SIBO [73]. SIBO may lead to malabsorption and vitamin and mineral deficiencies (e.g., vitamin B12, vitamin D, and iron) in severe cases [75,76].
The current gold standard for the diagnosis of SIBO is small bowel aspiration and culture. A bacterial colony count of > 103 colony-forming units per milliliter (CFU/mL) from culture confirms the diagnosis of SIBO. However, deep duodenal or jejunal aspiration and culture are invasive, time consuming, cumbersome, and expensive [77]. A recent well-designed duodenal aspirate and biopsy case-control study (n = 164) found that the presence of GI symptoms correlated with small bowel dysbiosis but not SIBO status (diagnosed based on duodenal culture), which indicated that alterations of bacterial composition in the small bowel may have more clinical relevance than bacterial overgrowth per se [78-80]. Another well-recognized method to diagnose SIBO is the use of glucose or lactulose breath tests. These breath tests are non-invasive, relatively easy to perform, and inexpensive. An increase in hydrogen ≥ 20 ppm from baseline within 90 minutes or methane > 10 ppm at any point is considered diagnostic of SIBO [77]. Based on these breath tests, the prevalence of SIBO in PD ranges from 25% to 67% [1,81-83]. However, these tests are often criticized for their relatively low sensitivity and specificity. In a meta-analysis of 14 studies, the pooled sensitivity/specificity of the glucose and lactulose breath tests were 54.5%/83.2% and 42.0%/70.6%, respectively [84]. Notably, two DB-RPCTs in PD found spontaneous changes in SIBO status in the placebo group, which supports caution in the reliability of SIBO breath tests and interpretation of SIBO studies in the field [44,85].
The cornerstone of SIBO management is treatment with broad-spectrum antibiotics, generally for 7–14 days [74]. The most well-established antibiotic is rifaximin, which has limited systemic absorption and side effects [74]. The treatment success rate with rifaximin was 70.8% in a meta-analysis of 32 clinical trials [86]. Other potentially helpful antibiotics are quinolone and azole antibiotics [86]. However, recurrence of SIBO is well recognized, with 12.6%, 27.5% and 43.7% relapse rates at 3, 6 and 9 months after treatment, respectively [87]. A small open-label SIBO eradication study in PD (n = 14) found a relapse rate of 42.9% at 6 months after SIBO eradication [83]. Although there is a lack of data in PD, re-treatment with antibiotics should be effective. Identifying and managing other SIBO risk factors or associated conditions (e.g., diabetes mellitus, hypothyroidism, prolonged usage of drugs, including opioids, TCAs, anticholinergics and PPIs) may reduce the chance of recurrence [74,88].
Implications of upper GI dysfunction on PD treatment
Levodopa is absorbed in the proximal small intestine [89]. Therefore, prolonged GE time may impair the delivery of levodopa to its absorption site and reduce its absorption because levodopa remaining in the stomach is converted to dopamine, which cannot cross the blood-brain barrier, and further impairs GE [1,4]. The resulting clinical features include levodopa response fluctuations, such as delayed ON state or dose failures. Notably, a recent small pilot study (n = 21) found high variability in gastric and small bowel transit time in patients with erratic motor fluctuations (i.e., delayed ON > 60 minutes, sudden ON/OFF state, or response failures), but with no significant difference compared to patients with easily predictable fluctuations [90]. Further studies are required to study the impact of upper gut motility on levodopa bioavailability and motor response complications in PD.
Gut microbial activities in the upper-mid gut may also significantly impact PD treatment because of their effects on levodopa absorption and bioavailability. For example, H. pylori infection with resultant gastroduodenitis and gastric hypochlorhydria may reduce levodopa absorption due to mucosal inflammation and the lower solubility of levodopa in an environment of reduced acidity [91,92]. However, the effect of H. pylori infection on levodopa pharmacokinetics [92,93] and the impact of H. pylori eradication on motor outcomes and fluctuations [44] are inconclusive based on the current mixed evidence. A 2020 DB-RPCT demonstrated no significant improvements in motor function or motor fluctuations following H. pylori eradication for up to 52 weeks [44]. Bacterial tyrosine decarboxylase converted levodopa to dopamine in the small intestine, which reduced levodopa absorption and bioavailability [94-96]. SIBO may result in increased abundances of tyrosine decarboxylase-producing bacteria and other enzymes that metabolize levodopa [95], which worsen motor outcomes and fluctuations [81,83]. However, a link between SIBO and increased peripheral inactivation of levodopa has yet to be convincingly demonstrated. Although SIBO eradication improved motor fluctuations in a small open-label study, levodopa pharmacokinetics were not improved [83].
Strategies to improve levodopa absorption include: 1) taking oral levodopa on an empty stomach, e.g. 1 hour before meals [1], 2) redistributing protein (e.g., concentrating protein intake during the last meal of the day, when the patient may be less physically active), 3) taking levodopa in combination with high-dose carbidopa [97], and 4) taking prokinetic agents, such as domperidone (discussed above). Strategies to bypass the GI tract in PD treatments include: 1) non-oral forms of dopamine replacement therapies (e.g., transdermal rotigotine, inhaled levodopa, subcutaneous apomorphine, or intrajejunal levodopa) and 2) surgical therapies, such as deep brain stimulation of the subthalamic nuclei [4].
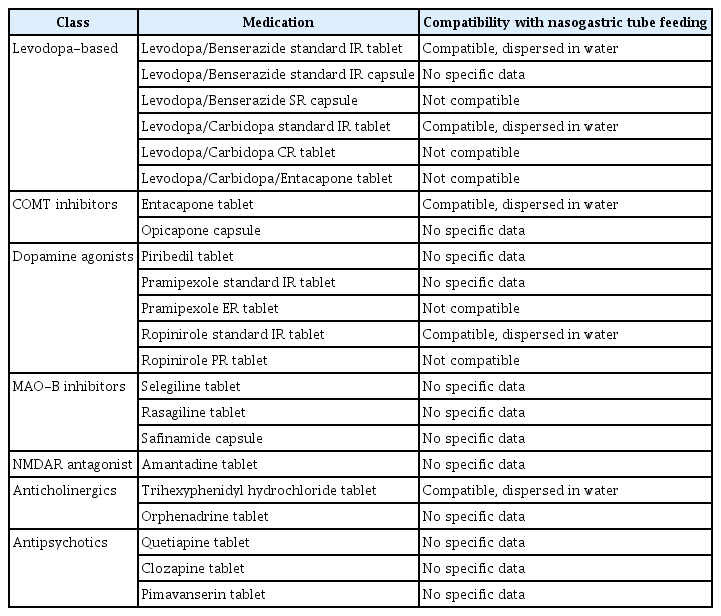
The literature on peri-operative management of PD patients undergoing surgery is limited. Timely peri-operative management strategies help minimize the risk of complications, such as akinetic crisis [98]. PD medications should be interrupted preand post-operatively for a minimum amount of time and resumed as soon as patients can tolerate oral intake. Non-oral forms of dopamine replacement therapies (e.g., transdermal rotigotine, inhaled levodopa, subcutaneous apomorphine, or intrajejunal levodopa) [4,99,100] may be considered as temporary or bridging therapy during the nil by mouth (NBM) period, especially for patients undergoing GI surgeries with prolonged NBM for complete bowel rest post-operatively. If administration of medication is needed via nasogastric (NG) tube, modifications of the PD treatment regimen may be necessary because certain oral formulations (e.g., capsules and extended-release preparations) are typically not recommended for NG tube administration (Table 2) [101]. Particular attention should be given to avoiding medications that have central dopamine-blocking properties, which may worsen motor symptoms, such as the commonly used anti-emetics metoclopramide and prochlorperazine, and domperidone and ondansetron may be used instead [99]. Likewise, neuroleptics such as haloperidol, risperidone, olanzapine, fluphenazine, and flupentixol are contraindicated, with quetiapine and clozapine being preferred when antipsychotic medication for the symptomatic control of psychosis or agitation is warranted [99].
LOWER GASTROINTESTINAL ISSUES IN PARKINSON’S DISEASE
Clinical Vignette: A 68-year-old man with PD has been having chronic constipation for the past 15 years and primarily relied on daily laxatives. His symptoms recently became worse. He has bowel openings twice weekly, often with a sense of incomplete evacuation. He spends one hour on the toilet in the mornings and has resorted to the use of manual evacuation at times. His medications included levodopa/carbidopa/entacapone (200/25/200 mg q.i.d.), quetiapine (25 mg ON), lactulose (15 ml ON), and bisacodyl (10 mg ON).
Clinicians often perceive constipation as infrequent bowel movements that are conventionally defined as < 3 bowel movements per week. However, patients may use the term “constipation” to indicate different lower GI symptoms that they are experiencing. Constipation represents a constellation of several highly individualized and troublesome symptoms, including lumpy or hard stools, bloating/abdominal pain, excessive straining, a sense of incomplete evacuation, and the need for manual evacuation [102]. Constipation symptoms correlate poorly with objective assessments. For example, studies have shown that symptoms, such as a sense of incomplete evacuation or the need for manual evacuation (which are often used as clinical surrogates for anorectal dysfunction), were weakly associated with the presence of dyssynergic defecation, and stool frequency was not a reliable marker for delayed colonic transit [103].
Chronic constipation—Slow transit vs. defecatory dyssynergia
Constipation is a very common symptom in PD, with a reported prevalence of up to 70%. 16 Notably, constipation is a prodromal symptom of PD that can predate the diagnosis (which is based primarily on the presence of cardinal motor features) by several decades [2]. It often occurs early in the disease course and tends to worsen as the disease progresses [16]. In addition to its impact on quality of life, the presence and/or severity of constipation has been associated with poorer prognosis in PD (e.g., poorer cognitive performance, loss of body fat, and faster progression to dementia and possibly death) [5,104-107]. Severe constipation in PD also rarely leads to life-threatening GI complications, such as intestinal pseudo-obstruction, volvulus, and intussusception [18].
Given their wide use, the ROME criteria for functional constipation (FC), which encompass colonic and anorectal symptoms, are useful to evaluate constipation in PD [108]. To diagnose FC, two of the following symptoms should be present for the last 3 months, with symptom onset at least six months before diagnosis: < 3 spontaneous bowel movements per week, lumpy or hard stools, straining, sensation of incomplete evacuation, sensation of anorectal obstruction/blockage, and/or the need to use manual maneuvers in > 25% of defecations [109]. FC has been reported as the most prevalent functional GI disorder in the community (6.6% to 11.7%) [110] and in primary care (11.7%) [111]. FC was the third most common condition in secondary care after FD and irritable bowel syndrome [112]. Importantly, patients with red flag symptoms, such as rectal bleeding, anemia, or a positive family history of colon cancer, should be considered for colonoscopy to exclude organic obstruction by neoplasm or inflammatory disease [113] Unexplained weight loss in the general population is also considered a reg flag symptom, but it is now recognized as a common symptom of PD itself [5,114].
Constipation in PD is likely due to slow colonic transit or anorectal dysfunction/defecatory dyssynergia (DD) (i.e., lack of relaxation of the puborectalis muscle or anal sphincters during defecation) [16,115]. The radiopaque marker test is the most commonly used test to measure colonic transit, but the test is cumbersome (patients are required to undergo several X-rays over 5–7 days) and lacks standardization [116]. More advanced methods, such as colonic transit scintigraphy and the WMC correlate well with the radiopaque marker test and have the additional advantage of estimating whole gut (including GE and small bowel) transit time [117,118]. However, both tests are expensive and not widely available. Digital rectal examination (DRE) is a valuable screening tool for DD, with a reported sensitivity and specificity of 71.3% and 76.1%, respectively [119]. During DRE, subjects are instructed to push and bear down as if to defecate, and an inability to contract the abdominal wall musculature, lack of propulsive force to push the examining finger out of the rectum, or insufficient anal sphincter relaxation and/or contraction of the puborectalis during simulated defecation suggest possible DD [103]. The balloon expulsion test is another simple and useful screening test for DD. Patients are instructed to evacuate a balloon filled with 50 mL of water or air, and an expulsion time > 1 minute is considered abnormal [103]. Suspected DD cases should ideally be confirmed using anorectal function and/or structure tests (e.g., anal manometry and barium or magnetic resonance [MR] defecography), but access to these tests is often limited. DD patients may have a lower recto-anal pressure gradient (with or without an increase in rectal pressure, accompanied by a paradoxical increase, rather than a decrease, in sphincter pressure) during evacuation on anal manometry recording, while paradoxical contraction of the puborectalis muscle and excessive perianal descent may be visualized on barium/MR defecography [120].
Colonic transit studies using radio-opaque marker tests revealed significant differences in colonic transit time between PD patients and controls, with approximately 80% of de novo and 85% of later-stage PD patients having prolonged colonic transit [7,16]. However, prolonged colonic transit time may result from DD and does not simply imply a diagnosis of slow-transit constipation. Combined evaluation of colonic transit and anorectal function in two studies of PD patients found a higher frequency of anal manometric abnormalities (70%–89%) compared to transit abnormalities (62%–66.7%), and most patients (57%–60%) had a combination of delayed colonic transit and anorectal dysfunction [115,121]. The positron emission tomography (PET) tracer [11C] donepezil has been used in recent research to quantify the density of acetylcholinesterase in peripheral organs as an in vivo measure of cholinergic/parasympathetic denervation [51,116]. Studies in PD showed reduced intestinal uptake of [11C] donepezil, and the colon was hypothesized to be affected earliest, followed by the small intestine and then the pancreas [51,116]. However, the PET signal did not correlate with constipation severity or GE time [51,116].
Management of chronic constipation in PD
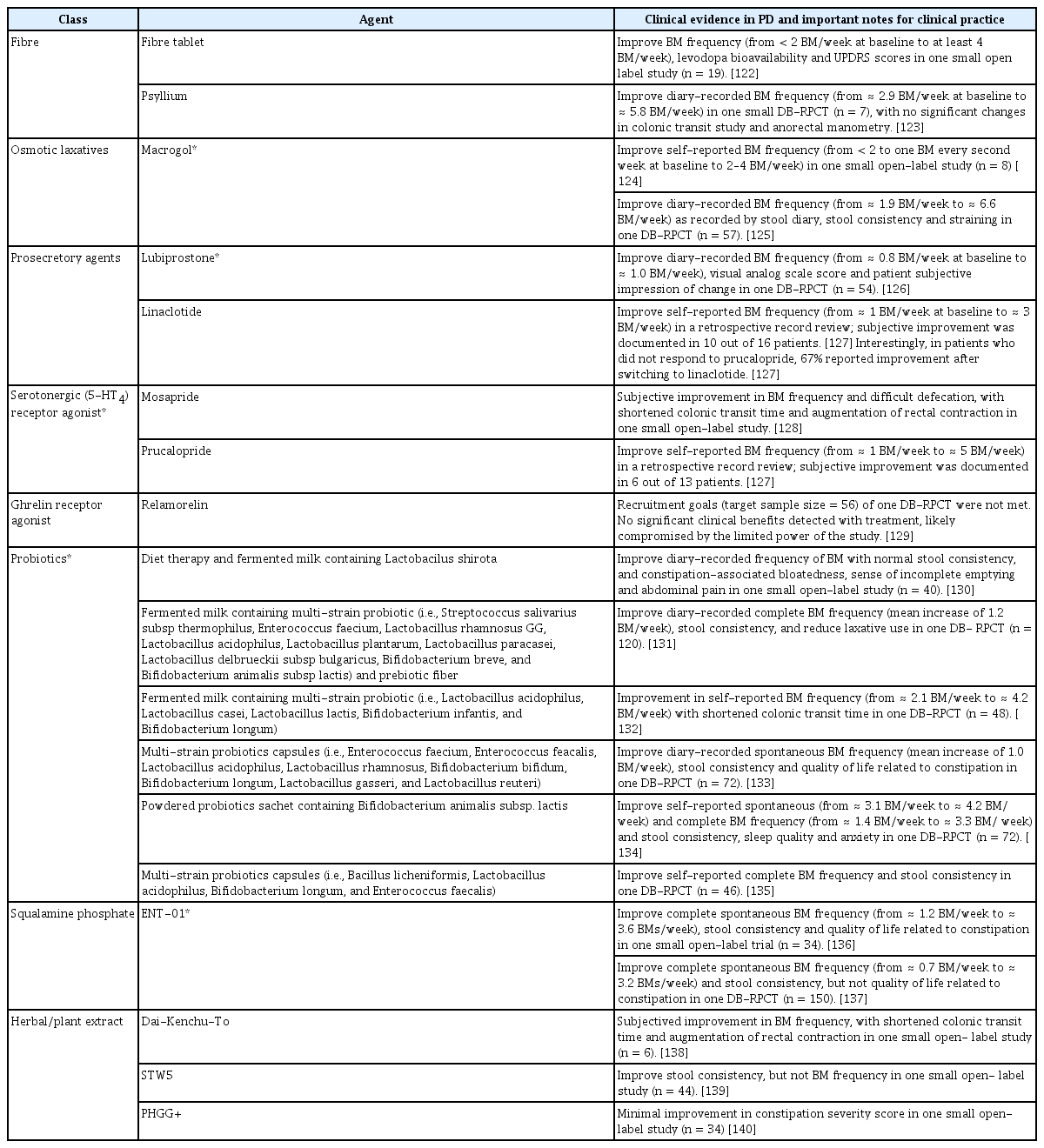
The need to determine the exact clinical constipation phenotype (slow colonic transit vs. DD) is often not considered essential in clinical practice when patients are responsive to lifestyle modifications or first-line therapies [103]. Most guidelines on constipation treatment recommend initial management using simple measures, such as increasing fiber (particularly soluble fiber) intake, fluid intake, and physical activity. Table 3 summarizes the pharmacological treatments for constipation in PD [122-140]. Two small studies (n = 7–19) demonstrated the potential benefits of high fiber tablets in the treatment of constipation in PD [122,123]. Notably, excessive fiber intake may cause or aggravate bloating in some patients. Osmotic laxatives (e.g., polyethylene glycol [PEG] or lactulose) are recommended as first-line therapies, and stimulant laxatives (e.g., bisacodyl, anthraquinones, or sodium picosulfate) may be considered in patients who do not respond to first-line therapies [103]. The suggestion that the chronic use of stimulant laxatives causes long-term problems (e.g., damage to the intestinal smooth muscle or the myenteric plexus with ‘cathartic colon’) is poorly documented, and more recent work suggests that the use of stimulant laxatives at recommended doses are unlikely to harm the colon [141]. Prokinetic (e.g., the serotonin 5-HT-4 agonist prucalopride) and prosecretory (e.g., lubiprostone or linaclotide) agents are recommended when patients have not responded to conventional laxatives [113].
Among the laxative options, only macrogol/PEG and lubiprostone improved stool frequency in PD in DB-RPCTs involving small cohorts of PD patients (n = 54–57) [125,126]. A small retrospective study reported potential benefits of linaclotide and prucalopride in the treatment of constipation in PD [127]. Notably, several DB-RPCTs demonstrated the efficacy of multistrain probiotics in different formulations (e.g., capsules, fermented milk, or powdered sachets) in improving stool frequency and consistency in PD patients with constipation [131-135]. Probiotics were administered for 4–12 weeks in these studies, and further research is needed to understand their longer-term effects, safety profile, and mechanisms of action in PD [142]. Macrogol and lubiprostone were designated “Likely Efficacious”, and probiotics and prebiotics fiber were designated “Efficacious” by the MDS EBM Committee on treatments for non-motor symptoms in PD [38]. A recent well-designed DB-RPCT (n = 150) demonstrated the efficacy of oral ENT-01 (a synthetic squalamine salt that effectively prevented the aggregation of alpha-synuclein and stimulated enteric neurons in pre-clinical studies) in improving constipation in PD, and it was well tolerated without any serious adverse events [136,137]. The potential benefits of fecal microbiota transplantation (FMT) have been reported in small case series (n = 1–11) [143-145]. Donor fecal microbiota was administered via colonoscopy in these studies, without major adverse events.
However, it is not uncommon for patients with PD to experience refractory constipation despite the use of various treatments [133]. Recognition of the constipation phenotype in these patients, especially the identification of DD using anorectal function tests, could improve the management strategy. Effective treatment for DD remains limited and challenging. Biofeedback is the preferred treatment because improvement is seen in up to 70%–80% of patients, and sustained improvement was demonstrated in long-term studies [146]. However, this therapy has not been specifically evaluated in PD. Suppositories may be useful in patients who are experiencing fecal impaction, which is often due to rectal sensorimotor dysfunction [147]. Approximately 65% of PD patients had rectal hyposensitivity in one small study [115]. Notably, phosphate enema should be avoided in older PD patients because of the risk of electrolyte disturbances. Two open-label studies (n = 10 and 18) in PD showed potential benefits of botulinum toxin injection into the puborectalis muscles with the objective of improving manometric parameters [148,149]. Evidence on intermittent subcutaneous apomorphine injection in the treatment of outlet-type constipation in PD remains limited [150,151].
Summary and conclusion
GI dysfunction is common in PD and manifests as a variety of different symptoms. Understanding the nature of GI symptoms faced by patients through careful clinical history and by using various diagnostic modalities helps provide a personalized management approach to these problems. An increasing number of useful treatment strategies are available, but these strategies are primarily based on evidence derived from the general population. Further well-designed clinical trials are required to properly evaluate their efficacy and safety in PD patients. Similar to the management of other aspects of PD, an interdisciplinary approach with joint neurology-gastroenterology input will provide more holistic care and benefit for patients. Importantly, management of GI dysfunction should be an integral part of PD treatment because symptomatic treatments have a strong positive impact on the overall function and quality of life in patients with PD.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.
Funding Statement
None
Author Contributions
Conceptualization: Ai Huey Tan. Data curation: Ai Huey Tan, Kee Huat Chuah, Yuan Ye Beh, Jie Ping Schee. Supervision: Ai Huey Tan, Kee Huat Chuah, Sanjiv Mahadeva, Shen-Yang Lim. Visualization: Ai Huey Tan, Yuan Ye Beh, Jie Ping Schee. Writing—original draft: Ai Huey Tan, Kee Huat Chuah. Writing—review & editing: all authors.