Restlessness with Manic Episodes due to Right Parietal Infarction
Article information
Abstract
Mood disorders following acute stroke are relatively common. However, restlessness with manic episodes has rarely been reported. Lesions responsible for post-stroke mania can be located in the thalamus, caudate nucleus, and temporal and frontal lobes. We present a patient who exhibited restlessness with manic episodes after an acute infarction in the right parietal lobe, and summarize the case reports involving post-stroke mania. The right parietal stroke causing mania in our case is a novel observation that may help us to understand the mechanisms underlying restlessness with mania following acute stroke.
Mood disorders following an acute stroke are relatively common. However, post-stroke mania has rarely been reported.1–17 Lesions responsible for post-stroke mania can be located in the thalamus, caudate nucleus, and temporal and frontal lobes.1–17 Here, we report on a patient who exhibited restlessness with manic episodes after an acute infarction in the right parietal lobe.
Case
A 75-year-old right-handed woman was admitted because of the abrupt development of mental confusion, left hemiparesis, and dysarthria. She was previously healthy. One day before admission, she developed weakness in her left arm and disorientation, followed 20 hours later by inappropriate speech, agitation, and dysarthria. She had a history of hypertension and diabetes, but no history of psychiatric illness. She had no family history of affective illness.
On admission, she showed partially impaired orientation to time, but had well-preserved orientation to place and person. The neurological examination revealed left homonymous hemianopsia, but no obvious motor or sensory deficits. She appeared agitated, and was talkative with an elated, euphoric mood. She also had a reduced need for sleep and pressured speech. She tended to touch the examiner while speaking. She showed no deficits in the line bisection and cancellation tasks, but was unable to copy an interlocked pentagon. She was also unable to draw a clock. Three days later, she developed motor weakness (grade 4) and hypesthesia in her left arm and face. Dysarthria developed along with persistence of her restlessness and manic features. The motor deficits and dysarthria were improved the next day, but the sensory deficit was unchanged.
Routine serologic testing and electrocardiography were all normal. Initial brain magnetic resonance imaging (MRI) with diffusion-weighted images (DWI) was performed on admission, which revealed an acute infarction in the right parietal lobe with subtle involvement of the posterior temporal area (Figure 1A). Magnetic resonance angiography (MRA) revealed an obstruction of portion M1 of the right middle cerebral artery (Figure 1B). Repeat MRI on the fourth hospital day after the motor deficits developed revealed that the infarction had increased in size (Figure 1C). Brain 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography (18F-FDG PET) performed 13 days after admission showed reduced FDG uptake in the right hemisphere, which was most severe in the right parietal lobe and left cerebellum (Figure 2A).
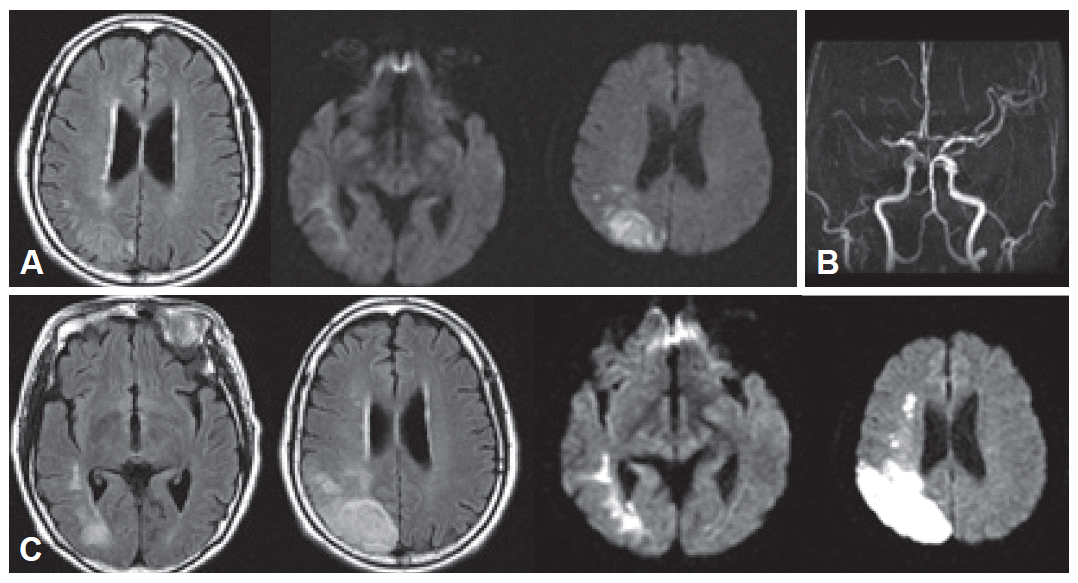
A: Brain MRI on admission shows an acute right parietal lobe infarction on FLAIR (left) and diffusion-weighted images (middle, right). B: Brain MRA shows obstruction of portion M1 of the right middle cerebral artery. C: Three days after admission, new neurological deficits developed. Follow-up brain MRI shows the infarct is larger.
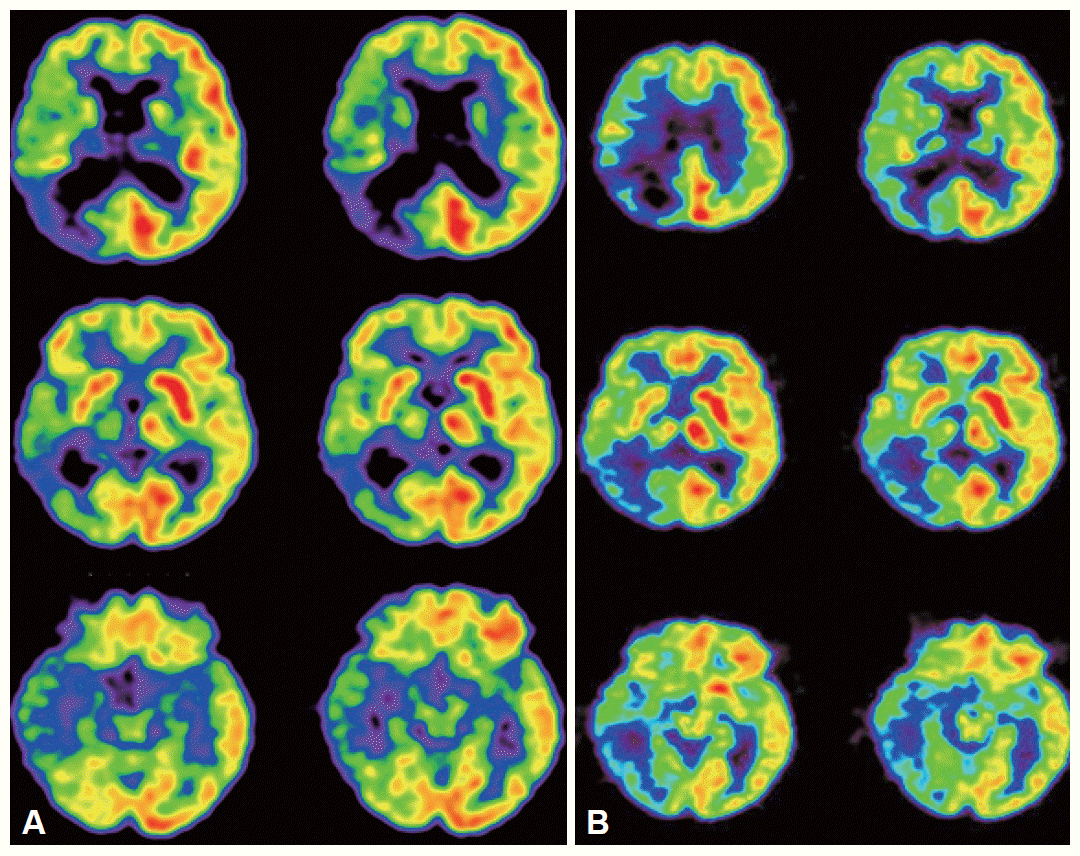
A: Brain 18F-FDG PET 13 days after admission shows diffusely decreased FDG uptake in the right hemisphere, most severely affecting the right parietal area. The FDG uptake in the right basal ganglia and thalamus is also decreased. B: At 13 months, repeat 18F-FDG PET shows improved FDG uptake in the right hemisphere, compared to the initial PET. 18F-FDG PET: [18F] fluoro-D-glucose prositron emission tomography.
When she was discharged 2 weeks after admission, her neurological deficits had improved. Her restlessness and manic symptoms had also partially alleviated without treatment. After 13 months, her neurological deficits and manic symptoms had resolved completely, except for the left-sided visual field defect. Repeated brain 18F-FDG PET showed that the previous reduction in FDG uptake in the right hemisphere had improved (Figure 2B).
Discussion
This patient had sudden-onset restlessness with manic symptoms, including agitated mood, irritability, euphoria, talkativeness, pressured speech, and decreased need for sleep. The close temporal relationship between the onset of her symptoms and ischemic stroke, along with the absence of a history of affective disorders, supports the hypothesis that her restlessness with mania was caused by acute infarction of the right parietal lobe.
An extensive literature search revealed 17 reports that described 29 patients who had post-stroke manic episodes. Of these, eleven patients developed manic symptoms concomitantly with their strokes. In the other cases, the temporal relationship between the stroke and mania was obscure, either with a fairly long latency or due to insufficient information. The lesions were located mostly in the right hemisphere. Only five patients had left-sided lesions. The reported anatomical locations of the strokes were the temporal and frontal cortex (13 patients), 1,2,5,6,15–17 thalamus (9 patients),3,5,8,9,11,13 caudate nucleus (2 patients),6 and ventral pontine region (2 patients).7 Some patients had infarctions in widespread regions involving the frontal, temporal, and parietal lobes. Our patient had an infarction that was mainly confined to the right parietal region on the initial brain MRI, although there was subtle involvement in the posterior temporal area. Post-stroke mania due to a localized infarction in the right parietal lobe has not previously been reported. One report described a case affecting the right parieto-occipital area involving the geniculocalcarine tract with agitation, hallucinations, and delusion, but the computed tomography result was not reported.18 Widespread reduction in right hemispheric activity was seen on the brain FDG PET. Although there was no structural damage (i.e., infarction), this may have been responsible for the restlessness and manic symptoms in this patient. However, the persistence of reduced activity in the right hemisphere, as shown on the repeated brain PET, along with a complete resolution of the manic symptoms, supports the possible relationship between the manic symptoms and the right parietal infarction.
Disinhibition syndrome, including restlessness and manic features, is usually caused by lesions in the orbitofrontal and basotemporal cortices of the right hemisphere.5,19 These areas are thought to inhibit the motor, instinctive, affective, and intellectual behaviors elaborated in the dorsal cortex selectively.19 Lesions in these areas or in their connections to the dorsal cortex could produce disinhibition syndrome. This may range from mildly inappropriate social behavior to full-blown mania.19 Caplan et al.20 observed that about half of the patients with infarcts of the inferior division of the right middle cerebral artery were in an agitated, confused state, showing hyperactivity, restlessness, and easy distractibility. Their motor and sensory deficits were usually mild and transient. These features are consistent with those of our patient. As Caplan et al.20 suggested, the inferior parietal lesions in our patient may interrupt the connections between the medial limbic cortex and superior parietal and frontal lobes, altering the emotional tone and affective behavior.
Notes
The authors have no financial conflicts of interest.