Usefulness of Diffusion-Weighted MRI for Differentiation between Parkinson’s Disease and Parkinson Variant of Multiple System Atrophy
Article information
Abstract
Background and Purpose:
Several studies have reported that diffusion-weighted imaging (DWI) is able to help discriminate a Parkinson variant of multiple system atrophy (MSA-p) from Parkinson’s disease (PD) on the basis of the increased regional apparent diffusion coefficient (rADC). We analyzed the usefulness of DWI by using the rADC for differential diagnosis between MSA-p and PD and investigated the correlation between the rADC value and clinical features of MSA-p and PD.
Methods:
Twelve patients with PD and 10 with MSA-p were studied. The rADC value was determined in different brain regions, including the dorsal putamen (DP) and middle cerebellar peduncles (MCP).
Results:
The rADC values of the DP showed a greater increase in MSA-p patients than in PD patients (p=0.03). MSA-p patients also presented increased rADC values of the MCP compared with PD patients (p=0.0001). In particular, the sensitivity, specificity and positive predictive values of the MCP rADC were higher than those of the DP rADC. However, DP and MCP rADC values were not correlated with clinical features in either MSA or PD patients.
Conclusions:
DWI discriminated between PD and MSA-p based on rADC values in DP and MCP. The MCP rADC value, in particular, could better discriminate MSA-p from PD.
Introduction
Parkinson’s disease (PD) and the Parkinson variant of multiple system atrophy (MSA-p) are neurodegenerative disorders. Despite consensus criteria for the diagnosis of PD1 and MSA,2 differential diagnosis remains a challenge for neurologists.
Since 1986, magnetic resonance imaging (MRI) has played an important role in diagnosing some neurodegenerative diseases3,4 and has also been used for differential diagnosis between PD and MSA-p. Because MR images in PD patients are generally normal, the diagnostic value of brain MRI for PD was limited.5 In advanced PD, however, MRI can reveal atrophy of the substantial nigra pars compacta (SNpc).6 In addition, MRI changes in PD include smudging towards the red nucleus (RN) of the anterior hypointensity, due to deposition of iron in the pars reticulate, which also involves the medial part of the cerebral peduncle.7 The MRI findings differentiating multiple system atrophy (MSA) from PD patients not only include a hyperintense rim at the putaminal edge and putaminal atrophy supratentorially but also infra-tentorial atrophy and signal change of the pons and middle cerebellar peduncle (MCP).5,8,9 Atrophy of the SNpc revealed by the smudging of the hypointensity towards the RN may also be seen in patients with MSA.6 Despite relatively high sensitivity and specificity of conventional MRI for differentiation between PD and MSA,8 it is very difficult to differentiate patients with both parkinsonian symptoms and atrophy of the SN.
Recently, interest in different MRI techniques, such as diffusion-weighted imaging (DWI), for differential diagnosis between PD and MSA-p has increased. DWI visualizes the random translation movement of water molecules in the tissue by applying diffusion-sensitized gradients between two radio-frequency pulses.10,11 DWI enables the assessment of the water apparent diffusion coefficient (ADC), a measure of tissue water diffusivity, and may detect changes in the microstructural integrity of nervous tissue earlier than conventional T1-or T2-weighted MRI.11 Regional ADC (rADC) depends on the interactions between water molecules and the chemical environment as well as the structural barriers at the cellular and subcellular level hindering their motion in vivo.12 A change of rADC is presumed to reflect ultrastructural tissue damage.13 Pathological processes that modify tissue integrity, as in neurodegenerative disorders, result in an increased rADC.12 The rADC value in DWI can help to distinguish MSA-p from PD by a high and normal putaminal rADC, respectively.14 Increased diffusivity has also been found in the caudate nucleus and globus pallidus in MSA-p compared to PD patients and controls, probably reflecting the spreading neurodegeneration in the basal ganglia.15 In addition increased rADC values in the MCP, which were usually found in MSA-c patients,16 were also found in the MSA-p patients.17
We compared the rADC values of DWI in the dorsal putamen (DP) and MCP to obtain a differential diagnosis between PD, MSA-p patients and normal controls. In addition, we analyzed to what extent MSA-p and PD could be differentiated. Lastly, we measured the correlation between clinical characteristics and rADC values in patients with both PD and MSA-p.
Subjects and Methods
Patients
Twenty-two patients, comprised of 12 patients with PD and 10 patients with MSA-p, and 10 age-matched healthy controls participated in this study. Diagnosis of PD and MSA-p was performed by an experienced movement disorder specialist using established diagnostic criteria.2 Disease severity and stages of all patients were quantified using the motor scale (part III) of the United Parkinson’s Disease Rating Scale (UPDRS)18 and the Hoehn and Yahr (H & Y) stage.19 None of the control subjects had structural MRI abnormalities or a history of neurological problems or psychiatric disease. None of the patients had a previous history of secondary parkinsonism by drugs or toxins. All patients undergo conventional brain MRI to exclude cerebrovascular disease such as cerebral infarction and other central nervous system (CNS) disorders. Informed consent was obtained from each patient and healthy volunteers.
MRI protocol
All subjects, including PD and MSA patients and normal controls were studied in a 1.5 tesla GE Signa MRI system (Echospeed, General Electrics Co, Milwaukee, WI, USA). Routine structural imaging included T1-weighted (repetition time [TR]/400 ms, echo time [TE]/9 ms, matrix 256×192 pixels) and T2-weighted (TR/2,000 ms, TE/120 ms, matrix 256×256 pixels) spin echo (SE) images (field of view=20×20 cm, 6 mm thickness, 1 mm gap, number of excitation 2). Routine T1- and T2-weighted SE images were reviewed by an experienced neuroradiologist blinded to the clinical diagnosis. In all cases, axial DWI was conducted using a SE-EPI sequence (TR/10,000 ms, TE/120 ms, field of view 24×24 cm, matrix 128×128 pixels, slice thickness 6 mm, slice gap 1 mm, scan acquisition time 40 s) with diffusion-sensitizing gradients applied along three x, y and z orthogonal directions, using b factors of 1,000 s/mm2.13 In addition, images (b factor of 0 s/mm2) without diffusion weighting were acquired and exhibited T2-contrast.
ADC data analysis
ADC maps were calculated on a pixel by pixel basis, assuming a signal dependence of the form S=S0 exp (-b×ADC), where S and So represent the signal intensities with (S) and without (S0) diffusion sensitization.13 The threshold of the ADC value was obtained at an arbitrary level to exclude pixels predominantly containing cerebrospinal fluid. The ADC maps were analyzed by the calculation of mean rADC values in each elliptical region of interest (ROI), which were placed in the left and right MCP as well as the DP. Maps of the mean rADC values were generated by calculating the mean of three x, y and z orthogonal directions.
Statistical analysis
Statistical comparison of each clinical item between all groups was performed with the χ2 test and Fisher’s exact test. Comparison of mean rADC values was performed with the Kruskal-Wallis test, followed by post hoc analysis corrected according to Bonferroni and Tukey for multiple comparisons. To evaluate the sensitivity, specificity and the positive and negative predictive value (PPV and NPV) of the mean rADC values for the DP and MCP for differential diagnosis, the optimal cut-off values of the mean rADCs were calculated by the receiver operator characteristic (ROC) curve approach. The relationship between the mean rADC and parkinsonian symptoms was evaluated using the Pearson correlation and/or Spearman correlation test. p<0.05 was considered statistically significant.
Results
Patients
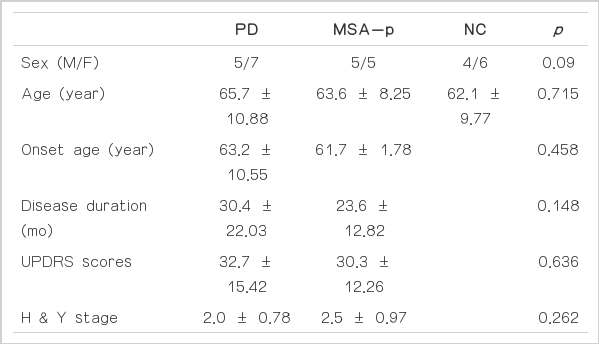
There was no difference in the sex distribution (p=0.09) or age (p=0.715) among PD, MSA-p and control patients. Furthermore, age at onset, disease duration, H & Y stage and UPDRS motor scores did not reveal a significant difference between PD and MSA-p patients (Table 1).
Diffusion-weighted imaging findings
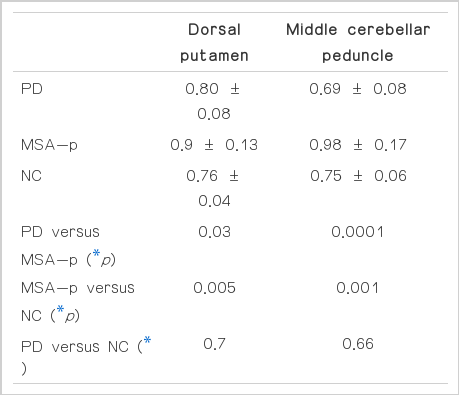
None of the PD patients had visually structural MRI abnormalities, whereas all of the MSA-p patients presented atrophy in the striatum, brainstem and/or cerebellum with small differences of degree. DP and MCP rADC values were significantly different among groups (DP rADC, p=0.02; MCP rADC, p=0.0001). The pair-wise comparison revealed high DP rADC values in MSA-p patients compared with PD patients (p=0.03) and control (p=0.005). Patients with MSA-p showed increased rADC values in the MCP patients compared with PD patients (p=0.0001) and controls (p=0.0001). However, DP and MCP rADC values were not significantly different between PD patients and controls (DP rADC, p= 0.7; MCP rADC, p=0.66, respectively)(Table 2).
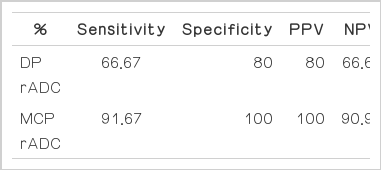
To calculate the sensitivity and specificity of the rADC values of the DP and MCP patients for differential diagnosis of MSA-p from PD, we used the cut-off values of 0.8225×10−3 mm2/s for DP rADC and 0.834×10−3 mm2/s for MCP rADC with ROC curve analysis. The DP rADC values differentiated MSA-p from PD with a sensitivity of 66.67%, a specificity of 80%, and a PPV of 80%. The MCP rADC values differentiated MSA-p from PD with a sensitivity of 91.67%, a specificity of 100% and a PPV of 100% (Table 3).
Discussion
Both DP and MCP rADC values were higher in the MSA-p patients than in PD patients or normal controls. Consistent with other recently published studies,17,20 our results show that rADC values can be used to differentially diagnose MSA-p from PD and healthy controls. The putamen and MCP of MSA-p patients are known to be representative regions for rADC values in the differential diagnosis of MSA-p and PD patients.14,17 As previously stated, DWI can detect loss of tissue integrity by showing regions with an increased ADC value due to increased water movement.21
We have focused on the identification of the rADC as a diagnostic marker helpful in the differential diagnosis between MSA-p and PD. In our study, MCP rADC values can distinguish MSA-p from PD patients with 91.67% sensitivity and 100% specificity, whereas DP rADC measurement showed a slightly lower sensitivity and specificity (66.67% vs. 80%, respectively). The positive and negative predictive values for the rADC of MCP were also higher than those of DP. To date, there have been no studies showing that DP and MCP rADC values can be used to discriminate MSA-p and PD. Our results suggest that MSA-p is related to a wider neurodegeneration over the whole brain, as compared to PD.
In a previous study, extrapyramidal symptoms, such as bradykinesia and rigidity, in MSA were shown to be associated with atrophy of the putamen, and the degree of atrophy in the pontocerebellar system was significantly correlated with the severity of truncal ataxia.9 We hypothesized, therefore, that an increased DP rADC value, which has been suggested to be related to neuronal degeneration or neuronal tissue atrophy of the putamen, would correlate with extrapyramidal symptoms, such as bradykinesia or rigidity, in MAS patients and that an increased MCP rADC value would be related to axial symptoms, such as standing from a sitting position, posture, gait or postural reflex, in the same patients. However, none of the DP and MCP rADC values were related to any clinical features in MSA patients or PD patients. In addition, DP and MCP rADC values were not correlated with the severity and duration of disease of MSA patients. There are a number of possible explanations for these results. First, “gait” in the UPDRS motor scale does not sufficiently reflect the major gait problem of MSA, which is usually ataxia. Second, the gait problem in MSA is associated with multiple lesions that extend from the MCP. Third, disease duration is slightly, but not significantly, longer in PD than MSA.
Lastly, the limitation of our study was that the number of PD and MSA patients was too small to reflect the diagnostic potential of our results. This point was not the reason that we were unable to find a correlation between DP and MCP rADC values with clinical scores in PD and MSA patients, respectively. The second limitation of our study was that multiple cerebral regions were not included in our study. In fact, many other structures such as the thalamus, globus pallidus, caudate nucleus, frontal or prefrontal white matter within the cortico-basal ganglia circuit were not analyzed. We believe that the second point was responsible for the negative correlation between rADC value and clinical features, especially in the MSA patients.
Acknowledgements
This work was supported by an Inje University Research Grant for 2007.