Novel Compound Heterozygous Mutations in the Pantothenate Kinase 2 Gene in a Korean Patient with Atypical Pantothenate Kinase Associated Neurodegeneration
Article information
Abstract
Pantothenate kinase-associated neurodegeneration (PKAN) is an autosomal recessive disorder that is characterized by mutations in the pantothenate kinase 2 gene (PANK2) and typical magnetic resonance imaging findings. We report a case of atypical PKAN presenting with generalized dystonia. Our patient had compound heterozygous mutations in the PANK2 gene, including mutation in exon 3 (p.D268G) and exon 4 (p.R330P). To our knowledge, this patient is the first to have the p.R330P mutation and the second to have the p.D268G mutation.
Pantothenate kinase-associated neurodegeneration (PKAN), the major form of neurodegeneration with brain iron accumulation (NBIA), is an autosomal recessive disorder that is characterized by mutations in the pantothenate kinase 2 gene (PANK2) and typical magnetic resonance imaging (MRI) findings.1 PKAN is classified into the classic form and an atypical form based on age at onset and rate of disease progression. The classic form usually presents in first decade of life and progresses rapidly to loss of ambulation within 10–15 years after onset. The clinical features of atypical PKAN are heterogeneous, but patients with this form commonly present in the second or third decade of life, and loss of ambulation occurs within 15–40 years after onset.2 We report the case of a patient with late-onset generalized dystonia who has a novel compound heterozygous mutations in the PANK2 gene.
Case Report
A 40-year-old man presented with involuntary movement of his right hand and left foot. The symptom started when he was approximately 35 years of age and had slowly worsened. Initially, his gait became unsteady. His disability progressed over the following years and was restricted to his right hand and left foot. The patient had been working as stonemason for the last 20 years. He had no significant medical history and no history of exposure to neuroleptic drugs, hypoxic environments, or toxic metabolites, except for the intermittent inhalation of silica dust at his workplace. There was no family history of neurological disorders.
On examination, the patient had dystonic posture of his right hand, which caused alternating dorsiflexion and neutralization with supination of the forearm and flexion of the metacarpophalangeal joint. He had fixed plantar flexion and intermittent inversion dystonia of his left foot. His gait was markedly spastic. The results of neuropsychological tests showed normal cognitive function. An ophthalmologic evaluation showed no Kayser-Fleischer rings, optic atrophy, or pigmentary retinopathy. The results of a nerve conduction study were normal. Serum ferritin and copper levels were normal, and a blood smear was negative for acanthocytes. The results of other laboratory tests, including serum electrolytes, erythrocyte sedimentation rate, thyroid function test, liver function test, autoimmune antibodies, ferritin, copper, ceruloplasmin, and a blood smear were normal, except for borderline-low 24-hour urine copper (10.2 μg/dL, normal 15–30 μg/dL).
A T1-weighted brain MRI study showed hyperintensity bilateral anteromedial globus pallidi (Figure 1A). A T2-weighted MRI showed hypointensity in the bilateral globus pallidi with hyperintense core, the typical “eye-of-the-tiger” sign (Figure 1B).
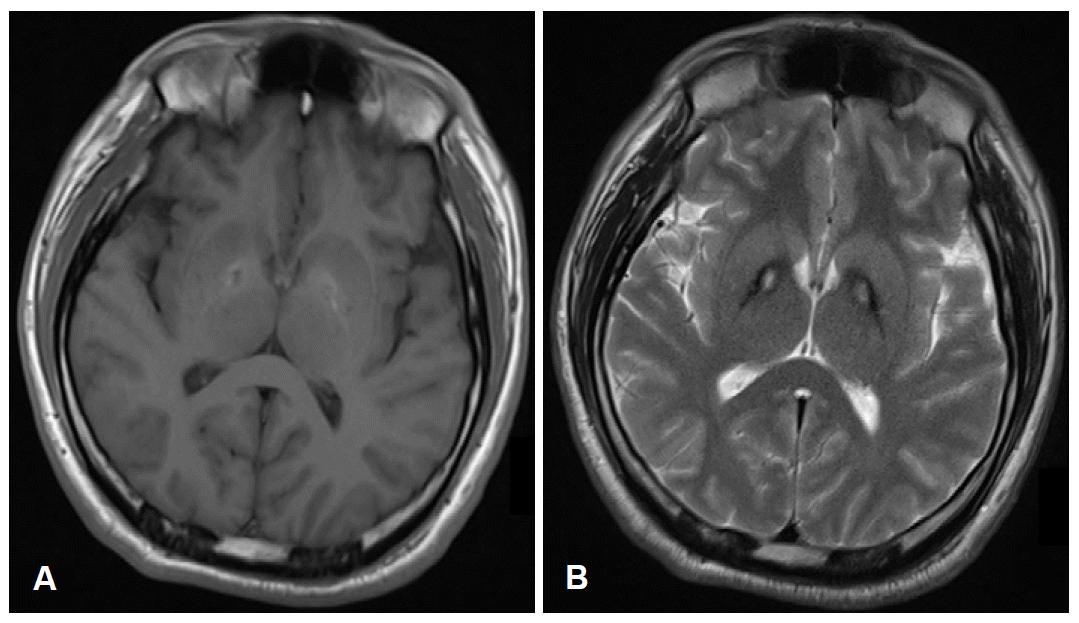
Axial brain MRI of the patient with compound heterozygous PANK2 mutations. A: T1-weighted brain MRI showed hyperintensity bilateral anteromedial globus pallidi. B: T2-weighted MRI showed hypointensity in the bilateral globus pallidi with hyperintense core, the typical “eye-of-the-tiger” sign.
Genomic DNA was extracted from peripheral blood, and polymerase chain reactions (PCR) were preformed. The DYT1 GAG deletion (delE302/303) was not found. Seven exons in the PANK2 gene were analyzed by PCR (Figure 2). Automated DNA sequence analyses revealed two single-base variants in exon 3 and exon 4. Both pathological variants result in changes to conserved amino acids in the PANK2 protein: Asp268→ Gly (p.D268G) and Arg330→Prp (p.R330P). To our knowledge, this is the first patient identified with the mutation p. R330P and the second patient with the mutation p.D268G.
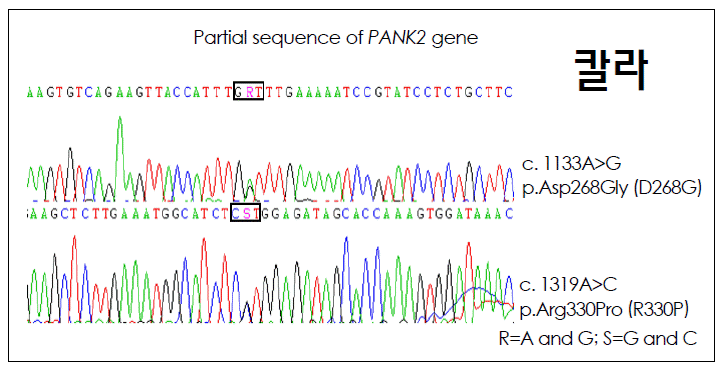
Automated DNA sequence analyses revealed two single-base variants in exon 3 and exon 4 in the PANK2 gene. Both pathological variants result in changes to conserved amino acids in the PANK2 protein: Asp268→Gly (p.D268G) and Arg330→Prp (p. R330P).
The dystonia of the patient’s right hand and left foot did not respond to levodopa/carbidopa (100/25 mg t.i.d). Treatment with diazepam (5 mg t.i.d) and trihexyphenidyl (2 mg t.i.d) slightly improved the dystonia. However, his dystonia worsened after one year, and he could not walk without a cane.
Discussion
Our patient had an atypical phenotype of the PANK2 gene: compound heterozygous mutations in exon 3 (p.D268G) and exon 4 (p.R330P). To our knowledge, our patient is the first to be identified with mutation p.R330P and the second to be identified with mutation p.D268G.
The onset of atypical PKAN is known to be 1st three decades (mean age 13.6).2 In a previous study, 23 patients with atypical PKAN were analyzed, and the mean age at onset was 13.7 years (range 1–28 years). Patients with atypical PKAN had variable clinical features, including parkinsonism, corticospinal tract involvement, speech disturbances such as palilalia, and psychiatric symptoms.3 Our patient presented with dystonia and gait disturbance, which are the predominant symptoms of classical PKAN.
The implication of the “eye-of-the-tiger” sign on a brain MRI is controversial.3,4 The “eye-of-the-tiger” sign on MRI is not pathognomic of PKAN, and some patients with PANK2 mutations do not have this sign and sometimes, it may appear only transiently. This sign has also been reported in corticobasal degeneration, progressive supranuclear palsy, anoxicischemic leukoencephalopathy, and early-onset levodopa-responsive parkinsonism.4 However, a previous study reported that all patients clinically suspected of NBIA with the “eye-of-tigersign” have mutations in PANK2 and that all patients without the typical MRI finding do not have these mutations.3 In our patient, the MRI finding and presenting symptoms were consistent with classic PKAN, while the age at onset and the absence of other features such as retinal degeneration, optic atrophy, and delayed development were consistent with atypical disease or other subtypes of NBIA.
Genetic studies identified compound heterozygous missense mutations (p.D268G, p.R330P) in exons 3 and 4 of PANK2. To our knowledge, this is the first description of the p.R330P mutation. The mutation p.D268G was reported previously in a Chinese patient with heterozygous missense mutations (p. D268G, p.I391N).5 Others have attempted to establish a correlation between the genotype and phenotype. Although the linkage between mutations and residual enzymatic activities related to the age of onset has been partially demonstrated, the phenotype is still difficult to predict based on the presence of PANK2 mutations.3,6–8 There were no overlapping clinical features between our patient and the Chinese patient with the p.D268G mutation, except for the typical MRI finding. The Chinese patient’s disease began at 17 years of age, and his predominant symptoms were tremor and rigidity. It is presumed that modifier effects of allelic genes and environmental factors played a roll in the phenotypic differences between these two patients.3
The PANK2 gene codes for pantothenate kinase, which is an essential regulatory enzyme in coenzyme A (CoA) biosynthesis, catalyzing the cytosolic phosphorylation of pantothenate (vitamin B5) the enzyme of the initial and rate-limiting step in CoA biosynthesis: the phosphorylation of pantothenate, N-pantothenoyl-cysteine and pantetheine.9 To compensate for the partial enzymatic deficiency in patients with PKAN, supplement of pantothenate (vitamin B5) have been tried without conclusive benefit.3 Previous case reports using pallidal deep-brain stimulation reported improvement of dystonia in patients with PKAN, but it was also restrictive effect.10 Further study is needed for early diagnosis of PKAN, correct prediction of disease progress, and rational treatment.
