Articles
- Page Path
- HOME > J Mov Disord > Volume 6(2); 2013 > Article
-
Original Article
Orthostatic and Supine Blood Pressures Are Associated with White Matter Hyperintensities in Parkinson Disease - Yoon-Sang Oh, Joong-Seok Kim, Kwang-Soo Lee
-
Journal of Movement Disorders 2013;6(2):23-27.
DOI: https://doi.org/10.14802/jmd.13006
Published online: October 30, 2013
Department of Neurology, College of Medicine, The Catholic University of Korea, Seoul, Korea
- Corresponding author: Joong-Seok Kim, MD, PhD, Department of Neurology, College of Medicine, The Catholic University of Korea, Seoul St. Mary’s Hospital, 222 Banpo-daero, Seocho-gu, Seoul 137-701, Korea, Tel +82-2-2258-6078, Fax +82-2-599-9686, E-mail neuronet@catholic.ac.kr
- The authors have no financial conflicts of interest.
Copyright © 2013 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 15,688 Views
- 102 Download
- 52 Crossref
ABSTRACT
-
Background and Purpose:
- Several reports on the elderly population have suggested that orthostatic hypotension is associated with white matter hyperintensities (WMH); however, little information is available on patients with Parkinson’s disease (PD).
-
Methods:
- We analyzed the association blood pressure profiles during tilt table testing with WMH scores in 117 patients with PD. WMH were rated using the semiquantitative visual rating system proposed by Scheltens et al.
-
Results:
- The presence of orthostatic hypotension was associated with increasing tendency of WMH score and the blood pressure changes during tilting and supine blood pressure were positively correlated with increasing WMH score.
-
Conclusions:
- This finding indicates that hemodynamic changes associated with orthostatic hypotension may be associated with white matter changes in patients with PD.
- Patients
- This study was approved by the local ethics committee, and each patient gave informed consent for participation.
- This study included 117 newly diagnosed patients. The clinical diagnosis of PD was made according to the UK Brain Bank criteria.11 At the time of the study, none of the patients had ever taken medications for PD. All patients underwent a detailed clinical evaluation, including laboratory tests for lipid profiles and serum homocysteine.
- At the time of study enrollment, all included patients were evaluated using the Unified Parkinson’s Disease Rating Scale (UPDRS) and the modified Hoehn and Yahr staging score. Tilt table testing was performed after discontinuing antihypertensive drugs for 7 days.
- Tilt table testing
- Patients were studied in a temperature-controlled clinical investigation room. Patients fasted the night before the test, except for a small amount of water, if they were thirsty and abstained from drinking alcohol or coffee the day before the study. Continuous electrocardiographic and non-invasive blood pressure monitoring was used in each patient (YM6000, Mediana Tech., San Antonio, TX, USA). After 30 min of supine rest, the test (20 min at 60°) was performed using the Manumed Special Tilt1-section (ENRAF NONIUS, Rotterdam, The Netherlands). By consensus, orthostatic hypotension was defined as a fall in blood pressure of at least 20 mm Hg systolic pressure and/or 10 mm Hg diastolic pressure between 2 and 5 minutes after tilt.12
- MRI and WMH rating
- All patients enrolled in the study underwent conventional MRI with a 3.0-T system (Magnetom Verio, 3T, Siemens). Axial T2 weighted images, complemented with coronal fluid attenuated recovery images, were used to rate the WMH.
- The MR images were rated using the semiquantitative visual rating system of Scheltens et al.13 This rating scale provides four sum scores in a semiquantitative way; periventricular hyperintensities (0–6), deep WMH (0–24), basal ganglia hyperintensities (0–30), and infratentorial hyperintensities (0–30). Periventricular hyperintensities were identified as continuous, confluent areas of high signal intensity adjacent to the anterior or posterior horns of the lateral ventricles (“cap”) and along the lateral ventricles (“bands”). Absence of lesions was a score of 0, ≤ 5 mm lesions was a score of 1, and a score of 2 was given for lesions > 5 mm. Hyperintensities > 10 mm were scored as lobar white matter changes. WMH, located in the deep and subcortical white matter, were separately rated in the frontal, temporal, parietal, and occipital regions according to the previously described criteria.13 These rating criteria were also applied to regions of the basal ganglia (caudate, putamen, globus pallidus, thalamus, and internal capsule) and infratentorial regions (cerebellum, midbrain, pons, and medulla).
- Data analysis
- The independent sample t-test or Pearson’s chi-square test was used to compare the differences between the groups categorized by the blood pressure response during orthostasis (non-OH and OH). Given the potential influence of several variables on the results of WMH, we also conducted group comparisons with an analysis of covariance (ANCOVA) including age, the presence of hypertension and diabetes, low density lipoprotein (LDL)-cholesterol, and serum homocysteine levels as confounding covariates. Patients were also divided into no to mild and severe WMHs groups based on two extremes by tertile distribution. Their BP results were analyzed by independent sample t-tests. The relationship between blood pressure profiles monitored during tilt table testing tilt and WMH were tested using Spearman’s rank correlation coefficients. The statistical analysis was performed with SPSS software version 15.0 (SPSS, Inc., Chicago, IL, USA). A p < 0.05 was considered significant.
Methods
- Among the 117 patients enrolled, 46 were men and 71 were women. The mean age at examination was 68.5 ± 10.1 years, and the mean duration of motor symptoms was 2.1 ± 1.0 years. The severities of parkinsonian motor symptoms were as follows: mean total UPDRS score was 24.7 ± 17.9 (part 1, 2.2 ± 2.3; part 2, 7.2 ± 5.8; part 3, 14.8 ± 10.7). Twenty-nine patients had hypertension (HBP), 24 had diabetes mellitus (DM), and 106 were non-smokers. The presence or absence of hypertension and diabetes, and current/past smoking habits did not affect cerebral WMH (HBP vs. no HBP group: 14.9 ± 8.6 vs. 11.9 ± 9.0, F = 1.295, p = 0.258; DM vs. no DM group: 15.8 ± 6.7 vs. 11.8 ± 9.3, F = 2.118, p = 0.148; non-smoker vs. ex-smoker vs. current-smoker 12.9 ± 8.9 vs. 9.8 ± 10.1 vs. 11.7 ± 8.4, F = 0.259, p = 0.772, by ANCOVA controlling for age, LDL-cholesterol and serum homocysteine). Higher LDL-cholesterol and serum homocysteine levels also did not correspond to increasing WMH scores (r = 0.004, p = 0.965 in LDL-cholesterol; r = 0.071, p = 0.494 in homocysteine by Spearman’s rank correlation).
- Clinical and laboratory profiles of the groups with and without orthostatic hypotension are presented in Table 1. Data from patients with and without orthostatic hypotension were similar for age, gender, duration of illness, severity of parkinsonian motor symptoms, and proportion of patients with hypertension and diabetes. Among the 42 patients with OH, 29 were clinically symptomatic (69.1%).
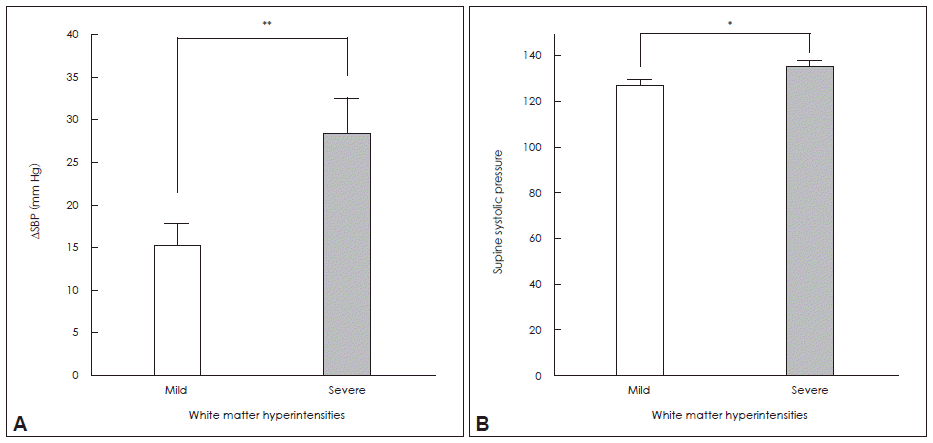
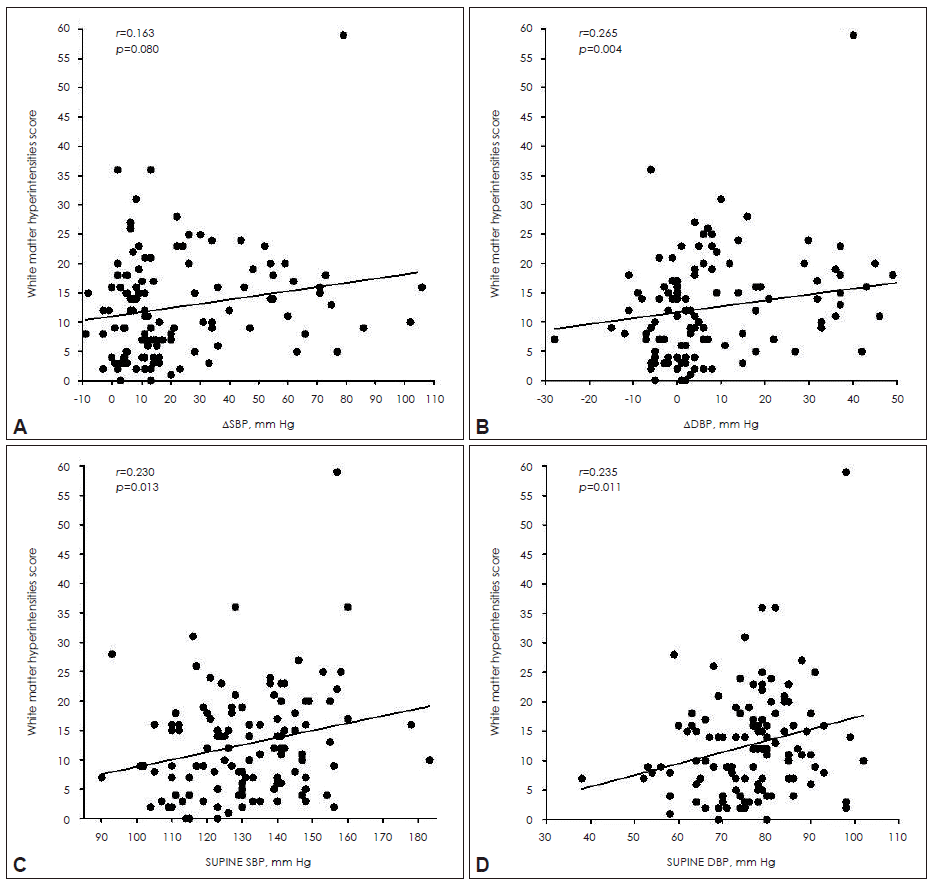
- The WMH scores were higher in the orthostatic hypotension group compared to those in the non-OH group after controlling for age, the presence of hypertension and diabetes, LDL-cholesterol and serum homocysteine. In addition, a trend of more WMH lesions in the supratentorial region of the OH group was also found (Table 2). On correlation analysis, orthostatic diastolic blood pressure change was positively correlated with white matter changes (Figure 1A and B). On the other hand, supine blood pressures were closely correlated with the WMH scores of the whole brain (Figure 1C and D). When comparing groups with extremes of WMHs, the severe WMH group had higher supine systolic BP and larger fall in BP during orthostasis (Figure 2).
Results
- The clinical importance of WMH has been emphasized frequently. Epidemiological investigations in healthy populations have shown that WMH predict the risk of cerebrovascular disease, cognitive dysfunction, and mortality.1 Advanced age, arterial hypertension, diabetes, and elevated serum homocysteine levels are risk factors for increasing WMH.2–4,14,15 However, these associations remain unclear in patients with PD.5,6 In this study, the presence or absence of cerebrovascular risk factors was not associated with WMH in patients with PD. This finding is consistent with previous studies and suggests that other nonvascular factors contribute to the vascular brain damage in patients with PD.
- Orthostatic hypotension is common in patients with PD and appears more frequently as disease progresses and influence subjective symptoms, quality of life, and disease treatment.16 Recent investigations in patients with multiple system atrophy indicate that supine systolic blood pressure and/or blood pressure changes during orthostasis are significantly and independently correlated with WMH.17,18 However, a similar phenomenon was not confirmed in patients with PD.18 Prior studies were not performed in patients with early stage and the enrolled patients were not free from drug effects. In this study, we enrolled only newly diagnosed patients with early stage PD who had not taken any dopaminergic medication. Because dopaminergic drugs can aggravate orthostatic hypotension, this result suggests that this association was independent from anti-parkinsonian treatment.
- We found that the orthostatic hypotension group had more severe WMH changes and that the differences in WMH were more significant in the deep white matter region. Severe orthostatic blood pressure drop was closely related to increasing WMH in tertile analysis. In addition, supine blood pressure may contribute in developing WMHs in PD.
- Although the hemodynamic consequences during orthostasis have not been clarified, it was reported that systemic blood flow is inversely associated with WMHs in the elderly.19 Because orthostatic hypotension leads to recurrent blood pressure drops, this could result in cerebral hypoperfusion and impact neurocirculatory failures, perhaps via cerebral small-vessel disease.20 Supine blood pressures can also contribute myriad episodes of cerebral hypo- and hyper-perfusion to white matter injuries. In addition to neurocirculatory insufficiency, degenerative processes associated with aging and disease itself may contribute to the generation of WMH in PD.
- In summary, our results indicate that hemodynamic changes associated with orthostatic hypotension may be associated with white matter changes in patients with PD. It provides important guideline for managing blood pressure in patients with PD.
Discussion

| Non-OH (n = 75) | OH (n = 42) | p | |
|---|---|---|---|
| Age (years) | 67.7 ± 11.1 | 69.8 ± 8.0 | 0.284 |
| No. of men* (n, %) | 25 (33.3) | 21 (50.0) | 0.077 |
| Disease duration (years) | 2.0 ± 0.9 | 2.2 ± 1.0 | 0.360 |
| Hypertension* (n, %) | 19 (25.3) | 10 (23.8) | 0.855 |
| Diabetes mellitus* (n, %) | 13 (17.3) | 11 (26.2) | 0.255 |
| Smoking status* (n, %) | |||
| Non-smoker | 69 (92.0) | 37 (88.1) | 0.689 |
| Ex-smoker | 4 (5.3) | 4 (9.5) | |
| Current smoker | 2 (2.7) | 1 (2.4) | |
| Lipid profiles (mg/dL) | |||
| Total cholesterol | 180.5 ± 32.2 | 178.0 ± 39.0 | 0.713 |
| LDL-cholesterol | 104.7 ± 27.5 | 106.9 ± 31.4 | 0.699 |
| H&Y stage | 1.9 ± 0.8 | 1.6 ± 0.6 | 0.160 |
| UPDRS | |||
| Total | 26.1 ± 19.5 | 22.1 ± 14.3 | 0.292 |
| Serum homocysteine level, μmol/L | 10.1 ± 5.1 | 10.2 ± 2.9 | 0.968 |
Values represent mean with standard deviation or numbers of patients with percentage in parenthesis. Analyses were performed by independent sample t-test or
* the chi-square test. OH: orthostatic hypotension, LDL: low density lipoprotein, H&Y: Hoehn and Yahr, UPDRS: Unified Parkinson’s Disease Rating Scale.
- 1. Debette S, Markus HS. The clinical importance of white matter hyper-intensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2010;341:c3666.ArticlePubMedPMC
- 2. Hajjar I, Quach L, Yang F, Chaves PH, Newman AB, Mukamal K, et al. Hypertension, white matter hyperintensities, and concurrent impairments in mobility, cognition, and mood: the Cardiovascular Health Study. Circulation 2011;123:858–865.ArticlePubMedPMC
- 3. de Leeuw FE, de Groot JC, Oudkerk M, Witteman JC, Hofman A, van Gijn J, et al. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain 2002;125(Pt 4):765–772.ArticlePubMed
- 4. van Dijk EJ, Breteler MM, Schmidt R, Berger K, Nilsson LG, Oudkerk M, et al. The association between blood pressure, hypertension, and cerebral white matter lesions: cardiovascular determinants of dementia study. Hypertension 2004;44:625–630.ArticlePubMed
- 5. Sławek J, Wieczorek D, Derejko M, Dubaniewicz M, Brockhuis B, Sitek E, et al. The influence of vascular risk factors and white matter hyperintensities on the degree of cognitive impairment in Parkinson’s disease. Neurol Neurochir Pol 2008;42:505–512.PubMed
- 6. Lee SJ, Kim JS, Yoo JY, Song IU, Kim BS, Jung SL, et al. Influence of white matter hyperintensities on the cognition of patients with Parkinson disease. Alzheimer Dis Assoc Disord 2010;24:227–233.ArticlePubMed
- 7. Goldstein DS. Dysautonomia in Parkinson’s disease: neurocardiological abnormalities. Lancet Neurol 2003;2:669–676.ArticlePubMedPMC
- 8. Sharabi Y, Goldstein DS. Mechanisms of orthostatic hypotension and supine hypertension in Parkinson disease. J Neurol Sci 2011;310:123–128.ArticlePubMedPMC
- 9. Park A, Stacy M. Non-motor symptoms in Parkinson’s disease. J Neurol 2009;256(Suppl 3):293–298.ArticlePubMed
- 10. Eguchi K, Kario K, Hoshide S, Hoshide Y, Ishikawa J, Morinari M, et al. Greater change of orthostatic blood pressure is related to silent cerebral infarct and cardiac overload in hypertensive subjects. Hypertens Res 2004;27:235–241.ArticlePubMed
- 11. Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 1988;51:745–752.ArticlePubMedPMC
- 12. Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res 1996;6:125–126.ArticlePubMed
- 13. Scheltens P, Barkhof F, Leys D, Pruvo JP, Nauta JJ, Vermersch P, et al. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci 1993;114:7–12.ArticlePubMed
- 14. Vermeer SE, van Dijk EJ, Koudstaal PJ, Oudkerk M, Hofman A, Clarke R, et al. Homocysteine, silent brain infarcts, and white matter lesions: The Rotterdam Scan Study. Ann Neurol 2002;51:285–289.ArticlePubMed
- 15. de Bresser J, Tiehuis AM, van den Berg E, Reijmer YD, Jongen C, Kappelle LJ, et al. Progression of cerebral atrophy and white matter hyperintensities in patients with type 2 diabetes. Diabetes Care 2010;33:1309–1314.ArticlePubMedPMC
- 16. Ziemssen T, Reichmann H. Cardiovascular autonomic dysfunction in Parkinson’s disease. J Neurol Sci 2010;289:74–80.ArticlePubMed
- 17. Lim TS, Lee PH, Kim HS, Yong SW. White matter hyperintensities in patients with multiple system atrophy. J Neurol 2009;256:1663–1670.ArticlePubMed
- 18. Umoto M, Miwa H, Ando R, Kajimoto Y, Kondo T. White matter hyperintensities in patients with multiple system atrophy. Parkinsonism Relat Disord 2012;18:17–20.ArticlePubMed
- 19. Jefferson AL, Tate DF, Poppas A, Brickman AM, Paul RH, Gunstad J, et al. Lower cardiac output is associated with greater white matter hyperintensities in older adults with cardiovascular disease. J Am Geriatr Soc 2007;55:1044–1048.ArticlePubMedPMC
- 20. Räihä I, Tarvonen S, Kurki T, Rajala T, Sourander L. Relationship between vascular factors and white matter low attenuation of the brain. Acta Neurol Scand 1993;87:286–289.ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Study protocol for the Heads-Up trial: a phase II randomized controlled trial investigating head-up tilt sleeping to alleviate orthostatic intolerance in Parkinson’s Disease and parkinsonism
Amber H. van der Stam, Nienke M. de Vries, Sharon Shmuely, Daan Smeenk, Joost H. Rutten, Ineke A. van Rossum, Susanne T. de Bot, Jurgen A. Claassen, Bastiaan R. Bloem, Roland D. Thijs
BMC Neurology.2024;[Epub] CrossRef - Parkinson's disease patients with absence of normal dipping status were more vulnerable to cognitive impairment from the early stages
Lanlan Chen, Li Jiang, Chenxin Wang, Tingting Qiao, Cancan Ma, Yingzhu Chen, Chunfeng Liu, Xin Wang, Yao Xu
Parkinsonism & Related Disorders.2024; 121: 106013. CrossRef - The Relationship among the Neurovascular Changes, Clinical Phenotypes, and White Matter Impairment in Parkinson’s Disease
司晨 李
Advances in Clinical Medicine.2024; 14(04): 1908. CrossRef - Association Between Gait and Dysautonomia in Patients With De Novo Parkinson’s Disease: Forward Gait Versus Backward Gait
Seon-Min Lee, Mina Lee, Eun Ji Lee, Rae On Kim, Yongduk Kim, Kyum-Yil Kwon
Journal of Movement Disorders.2023; 16(1): 59. CrossRef - Association Between Orthostatic Hypotension and Dementia in Patients With Parkinson Disease and Multiple System Atrophy
Iñigo Ruiz Barrio, Yasuo Miki, Zane T. Jaunmuktane, Thomas Warner, Eduardo De Pablo-Fernandez
Neurology.2023;[Epub] CrossRef - Central retinal microvasculature damage is associated with orthostatic hypotension in Parkinson’s disease
Jong Hyeon Ahn, Min Chae Kang, Dongyoung Lee, Jin Whan Cho, Kyung-Ah Park, Jinyoung Youn
npj Parkinson's Disease.2023;[Epub] CrossRef - Interventions for orthostatic hypotension in Parkinson's disease: a systematic review and network meta-analysis
Kunshan Li, Luyi Wu, Xuejun Cui, Wei Zhang, Jun Ji, Yiwen Wu, Zhaoqin Wang, Huirong Liu, Huangan Wu, Lu Zhu
Cochrane Database of Systematic Reviews.2023;[Epub] CrossRef - Autonomic control of heart and vessels in patients with very early stage of Parkinson disease
J Oleksakova, M Javorka, B Czippelova, N Mazgutova, M Grofik, L Babalova, P Skacik, E Kurca
Physiological Measurement.2023; 44(5): 054002. CrossRef - The mechanism of impaired delayed recall verbal memory function in Parkinson's disease with orthostatic hypotension: a multiple imaging study
Xiaofan Xue, Anqi Huang, Jingrong Zeng, Haixia Song, Yingqi Xing, Piu Chan, Erhe Xu, Lichun Zhou
Frontiers in Neurology.2023;[Epub] CrossRef - Cognitive Deficits in Parkinson's Disease Are Associated with Neuronal Dysfunction and Not White Matter Lesions
Nils Schröter, Tobias Bormann, Michel Rijntjes, Ganna Blazhenets, Raissa Berti, Bastian E.A. Sajonz, Horst Urbach, Cornelius Weiller, Philipp T. Meyer, Alexander Rau, Lars Frings
Movement Disorders Clinical Practice.2023; 10(7): 1066. CrossRef - Orthostatic hypotension and its association with cerebral small vessel disease in a memory clinic population
Julia H.I. Wiersinga, Hanneke F.M. Rhodius-Meester, Frank J. Wolters, Marijke C. Trappenburg, Afina W. Lemstra, Frederik Barkhof, Mike J.L. Peters, Wiesje M. van der Flier, Majon Muller
Journal of Hypertension.2023; 41(11): 1738. CrossRef - Mild cognitive impairment in patients with Parkinson´s disease and the analysis of associated factors
Esma Kobak Tur, Buse Cagla Ari
Neurological Research.2023; 45(12): 1161. CrossRef - REM sleep behavioral disorder may be an independent risk factor for orthostatic hypotension in Parkinson’s disease
Kangfu Yin, Chuanbin Zhou, Yongyun Zhu, Weifang Yin, Lei Yin, Bin Liu, Hui Ren, Zhong Xu, Xinglong Yang
Aging Clinical and Experimental Research.2022; 34(1): 159. CrossRef - Parkinsonism and cerebrovascular disease
Manisha Narasimhan, Raymond Schwartz, Glenda Halliday
Journal of the Neurological Sciences.2022; 433: 120011. CrossRef - Arterial Blood Pressure Variability and Other Vascular Factors Contribution to the Cognitive Decline in Parkinson’s Disease
Anna Pierzchlińska, Magdalena Kwaśniak-Butowska, Jarosław Sławek, Marek Droździk, Monika Białecka
Molecules.2021; 26(6): 1523. CrossRef - Adverse effects of hypertension, supine hypertension, and perivascular space on cognition and motor function in PD
Na-Young Shin, Yae Won Park, Sang-Won Yoo, Ji-Yeon Yoo, Yangsean Choi, Jinhee Jang, Kook-Jin Ahn, Bum-soo Kim, Joong-Seok Kim
npj Parkinson's Disease.2021;[Epub] CrossRef - Association of Orthostatic Hypotension With Cerebral Atrophy in Patients With Lewy Body Disorders
Andrea Pilotto, Alberto Romagnolo, Andrea Scalvini, Mario Masellis, Yasushi Shimo, Laura Bonanni, Richard Camicioli, Lily L. Wang, Alok K. Dwivedi, Katherine Longardner, Federico Rodriguez-Porcel, Mark DiFrancesco, Joaquin A. Vizcarra, Elisa Montanaro, Si
Neurology.2021;[Epub] CrossRef - Small vessel disease pathological changes in neurodegenerative and vascular dementias concomitant with autonomic dysfunction
Yoshiki Hase, Tuomo M. Polvikoski, Michael J. Firbank, Lucinda J. L. Craggs, Emily Hawthorne, Charlotte Platten, William Stevenson, Vincent Deramecourt, Clive Ballard, Rose Anne Kenny, Robert H. Perry, Paul Ince, Roxana O. Carare, Louise M. Allan, Karen H
Brain Pathology.2020; 30(1): 191. CrossRef - Neurovascular unit dysregulation, white matter disease, and executive dysfunction: the shared triad of vascular cognitive impairment and Alzheimer disease
Alexander Levit, Vladimir Hachinski, Shawn N. Whitehead
GeroScience.2020; 42(2): 445. CrossRef - The impact of supine hypertension on target organ damage and survival in patients with synucleinopathies and neurogenic orthostatic hypotension
Jose-Alberto Palma, Gabriel Redel-Traub, Angelo Porciuncula, Daniela Samaniego-Toro, Patricio Millar Vernetti, Yvonne W. Lui, Lucy Norcliffe-Kaufmann, Horacio Kaufmann
Parkinsonism & Related Disorders.2020; 75: 97. CrossRef - White Matter Hyperintensities in the Synucleinopathies: Orthostatic Hypotension, Supine Hypertension, or Both?
Horacio Kaufmann, Jose‐Alberto Palma
Movement Disorders Clinical Practice.2020; 7(6): 595. CrossRef - White Matter Hyperintensities Mediate Impact of Dysautonomia on Cognition in Parkinson's Disease
Mahsa Dadar, Seyed‐Mohammad Fereshtehnejad, Yashar Zeighami, Alain Dagher, Ronald B. Postuma, D. Louis Collins
Movement Disorders Clinical Practice.2020; 7(6): 639. CrossRef - Blood pressure lability is associated with subcortical atrophy in early Parkinson's disease
Sang-Won Yoo, Eunkyeong Yun, Mirim Bang, Uicheul Yoon, Ji-Yeon Yoo, Kwang-Soo Lee, Na-Young Shin, Joong-Seok Kim
Journal of Hypertension.2020; 38(10): 2043. CrossRef - Orthostatic hypotension, dizziness, neurology outcomes, and death in older adults
Stephen P. Juraschek, W.T. Longstreth, Oscar L. Lopez, John S. Gottdiener, Lewis A. Lipsitz, Lewis H. Kuller, Kenneth J. Mukamal
Neurology.2020;[Epub] CrossRef - Cross-sectional and longitudinal associations between total and regional white matter hyperintensity volume and cognitive and motor function in Parkinson's disease
Vincent Pozorski, Jennifer M. Oh, Ozioma Okonkwo, Stephanie Krislov, Amy Barzgari, Frances Theisen, Jitka Sojkova, Barbara B. Bendlin, Sterling C. Johnson, Catherine L. Gallagher
NeuroImage: Clinical.2019; 23: 101870. CrossRef - Magnetic Resonance Imaging–Visible Perivascular Spaces in Basal Ganglia Predict Cognitive Decline in Parkinson's Disease
Yae Won Park, Na‐Young Shin, Seok Jong Chung, Jiwoong Kim, Soo Mee Lim, Phil Hyu Lee, Seung‐Koo Lee, Kook Jin Ahn
Movement Disorders.2019; 34(11): 1672. CrossRef - Orthostatic hypotension in Parkinson disease
Ylva Hivand Hiorth, Kenn Freddy Pedersen, Ingvild Dalen, Ole-Bjørn Tysnes, Guido Alves
Neurology.2019;[Epub] CrossRef - Dysautonomia is associated with structural and functional alterations in Parkinson disease
Seok Jong Chung, Youn Jung Bae, Suhnyoung Jun, Han Soo Yoo, Seung Woo Kim, Yang Hyun Lee, Young H. Sohn, Seung-Koo Lee, Joon-Kyung Seong, Phil Hyu Lee
Neurology.2019;[Epub] CrossRef - Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the American Autonomic Society (AAS) and the European Federation of Autonomic Societies (EFAS)
Alessandra Fanciulli, Jens Jordan, Italo Biaggioni, Giovanna Calandra–Buonaura, William P. Cheshire, Pietro Cortelli, Sabine Eschlboeck, Guido Grassi, Max J. Hilz, Horacio Kaufmann, Heinz Lahrmann, Giuseppe Mancia, Gert Mayer, Lucy Norcliffe–Kaufmann, Ann
Clinical Autonomic Research.2018; 28(4): 355. CrossRef - Reverse blood pressure dipping as marker of dysautonomia in Parkinson disease
Valeria Milazzo, Cristina Di Stefano, Fabrizio Vallelonga, Gabriele Sobrero, Maurizio Zibetti, Alberto Romagnolo, Aristide Merola, Alberto Milan, Alberto J. Espay, Leonardo Lopiano, Franco Veglio, Simona Maule
Parkinsonism & Related Disorders.2018; 56: 82. CrossRef - White matter hyperintensities are linked to future cognitive decline in de novo Parkinson's disease patients
Mahsa Dadar, Yashar Zeighami, Yvonne Yau, Seyed-Mohammad Fereshtehnejad, Josefina Maranzano, Ronald B. Postuma, Alain Dagher, D. Louis Collins
NeuroImage: Clinical.2018; 20: 892. CrossRef - Rapid Systolic Blood Pressure Changes After Standing Up Associate With Impaired Physical Performance in Geriatric Outpatients
Arjen Mol, Esmee M. Reijnierse, Marijke C. Trappenburg, Richard J. A. van Wezel, Andrea B. Maier, Carel G. M. Meskers
Journal of the American Heart Association.2018;[Epub] CrossRef - Central hemodynamics and arterial stiffness in idiopathic and multiple system atrophy
Klaas Franzen, Sabine Fliegen, Jelena Koester, Rafael Campos Martin, Günther Deuschl, Michael Reppel, Kai Mortensen, Susanne A. Schneider
Journal of Neurology.2017; 264(2): 327. CrossRef - Cardiovascular Autonomic Dysfunction in Patients with Drug-Induced Parkinsonism
Joong-Seok Kim, Dong-Woo Ryu, Ju-Hee Oh, Yang-Hyun Lee, Sung-Jin Park, Kipyung Jeon, Jong-Yun Lee, Seong Hee Ho, Jungmin So, Jin Hee Im, Kwang-Soo Lee
Journal of Clinical Neurology.2017; 13(1): 15. CrossRef - Association of arterial stiffness with cognition in patients with Lewy body disorder
Dong-Woo Ryu, Joong-Seok Kim, Jee-Eun Lee, Jeong-Wook Park, Yoon-Sang Oh, Jae-Young An, Kwang-Soo Lee
Neurological Sciences.2017; 38(7): 1307. CrossRef - Microstructural integrity of white matter tracts amongst older fallers: A DTI study
Yoke Queen Wong, Li Kuo Tan, Pohchoo Seow, Maw Pin Tan, Khairul Azmi Abd Kadir, Anushya Vijayananthan, Norlisah Ramli, Gianluigi Forloni
PLOS ONE.2017; 12(6): e0179895. CrossRef - Cerebral autoregulation is preserved in multiple sclerosis patients
Daniel Ferreira, Pedro Castro, Gonçalo Videira, João Pedro Filipe, Rosa Santos, Maria José Sá, Elsa Azevedo, Pedro Abreu
Journal of the Neurological Sciences.2017; 381: 298. CrossRef - Long-term Levodopa Treatment Accelerates the Circadian Rhythm Dysfunction in a 6-hydroxydopamine Rat Model of Parkinson's Disease
Si-Yue Li, Ya-Li Wang, Wen-Wen Liu, Dong-Jun Lyu, Fen Wang, Cheng-Jie Mao, Ya-Ping Yang, Li-Fang Hu, Chun-Feng Liu
Chinese Medical Journal.2017; 130(9): 1085. CrossRef - Arterial Stiffness and Cardiovascular Autonomic Dysfunction in Patients with Parkinson's Disease
Joong-Seok Kim, Si-Hoon Lee, Yoon-Sang Oh, Jeong-Wook Park, Jae-Young An, Hyun-Seok Choi, Kwang-Soo Lee
Neurodegenerative Diseases.2017; 17(2-3): 89. CrossRef - Cardiovascular autonomic dysfunctions in elderly patients with essential tremor: comparison with healthy controls
Joong-Seok Kim, Yoon-Sang Oh, Hyung-Eun Park, Si-Hoon Lee, Jeong-Wook Park, In-Uk Song, Jae-Young An, Hun-Jun Park, Byung-Chul Son, Kwang-Soo Lee
Neurological Sciences.2016; 37(5): 711. CrossRef - Orthostatic hypotension and cardiac sympathetic denervation in Parkinson disease patients with REM sleep behavioral disorder
Joong-Seok Kim, Hyung-Eun Park, Yoon-Sang Oh, Si-Hoon Lee, Jeong-Wook Park, Byung-chul Son, Kwang-Soo Lee
Journal of the Neurological Sciences.2016; 362: 59. CrossRef - Supine hypertension in Parkinson’s disease and multiple system atrophy
Alessandra Fanciulli, Georg Göbel, Jean Pierre Ndayisaba, Roberta Granata, Susanne Duerr, Stefano Strano, Carlo Colosimo, Werner Poewe, Francesco E. Pontieri, Gregor K. Wenning
Clinical Autonomic Research.2016; 26(2): 97. CrossRef - Orthostatic hypotension and cognitive impairment in Parkinson's disease: Causation or association?
Claire McDonald, Julia L. Newton, David J. Burn
Movement Disorders.2016; 31(7): 937. CrossRef - Cardiovascular Autonomic Dysfunction in Mild and Advanced Parkinson’s Disease
Joong-Seok Kim, Si-Hoon Lee, Yoon-Sang Oh, Jeong-Wook Park, Jae-Young An, Sung-Kyung Park, Si-Ryung Han, Kwang-Soo Lee
Journal of Movement Disorders.2016; 9(2): 97. CrossRef - Autonomic Nervous System Dysfunction in Patients With Parkinson Disease Having Depression
Hyung-Eun Park, Joong-Seok Kim, Yoon-Sang Oh, In-Seok Park, Jeong-Wook Park, In-Uk Song, Kwang-Soo Lee
Journal of Geriatric Psychiatry and Neurology.2016; 29(1): 11. CrossRef - Vascular cognitive impairment, a cardiovascular complication
Adiukwu Frances, Ofori Sandra, Ugbomah Lucy
World Journal of Psychiatry.2016; 6(2): 199. CrossRef - Cardiovascular complications in patients with autonomic failure
Valeria Milazzo, Cristina Di Stefano, Alberto Milan, Agnese Ravera, Gabriele Sobrero, Luca Sabia, Franco Veglio, Simona Maule
Clinical Autonomic Research.2015; 25(3): 133. CrossRef - 123I-MIBG myocardial scintigraphy and neurocirculatory abnormalities in patients with dementia with Lewy bodies and Alzheimer's disease
Joong-Seok Kim, Hyung-Eun Park, Yoon-Sang Oh, In-Uk Song, Dong-Won Yang, Jeong-Wook Park, Kwang-Soo Lee
Journal of the Neurological Sciences.2015; 357(1-2): 173. CrossRef - Diagnosing and treating neurogenic orthostatic hypotension in primary care
Louis Kuritzky, Alberto J. Espay, Jeffrey Gelblum, Richard Payne, Eric Dietrich
Postgraduate Medicine.2015; 127(7): 702. CrossRef - Increased CSF biomarkers of angiogenesis in Parkinson disease
Shorena Janelidze, Daniel Lindqvist, Veronica Francardo, Sara Hall, Henrik Zetterberg, Kaj Blennow, Charles H. Adler, Thomas G. Beach, Geidy E. Serrano, Danielle van Westen, Elisabet Londos, M. Angela Cenci, Oskar Hansson
Neurology.2015; 85(21): 1834. CrossRef - Orthostatic hypotension, cerebral hypoperfusion, and visuospatial deficits in Lewy body disorders
Andrew D. Robertson, Michelle A. Messner, Zahra Shirzadi, Galit Kleiner-Fisman, Joyce Lee, Julia Hopyan, Anthony E. Lang, Sandra E. Black, Bradley J. MacIntosh, Mario Masellis
Parkinsonism & Related Disorders.2015;[Epub] CrossRef - Orthostatic Hypotension and Cognitive Impairment in <i>De Novo</i> Patients with Parkinson’s Disease
Hyo-Jin Bae, Jun-Ho Lim, Sang-Myung Cheon
Journal of Movement Disorders.2014; 7(2): 102. CrossRef
Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite