Articles
- Page Path
- HOME > J Mov Disord > Volume 14(3); 2021 > Article
-
Brief communication
Clinical and Imaging Profile of Patients with Joubert Syndrome -
Bharath Kumar Surisetti1*
 , Vikram Venkappayya Holla1*
, Vikram Venkappayya Holla1* , Shweta Prasad1,2
, Shweta Prasad1,2 , Koti Neeraja1
, Koti Neeraja1 , Nitish Kamble1
, Nitish Kamble1 , Ravi Yadav1
, Ravi Yadav1 , Pramod Kumar Pal1
, Pramod Kumar Pal1
-
Journal of Movement Disorders 2021;14(3):231-235.
DOI: https://doi.org/10.14802/jmd.21066
Published online: September 16, 2021
1Department of Neurology, National Institute of Mental Health and Neurosciences (NIMHANS), Karnataka, India
2Department of Clinical Neurosciences, National Institute of Mental Health and Neurosciences (NIMHANS), Karnataka, India
- Corresponding author: Pramod Kumar Pal, MD, DNB, DM, FRCP Department of Neurology, National Institute of Mental Health and Neurosciences (NIMHANS), Hosur Road, Bengaluru, Karnataka 560029, India / Tel: +91-80-26995147 / Fax: +91-80-26564830 / E-mail: palpramod@hotmail.com
- *These authors contributed equally to this work.
Copyright © 2021 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objective
- Joubert syndrome (JS) is a rare syndrome characterized by ataxia and the molar tooth sign (MTS) on imaging. The present study aims to explore the clinical and radiological features in a cohort of patients with JS.
-
Methods
- This was a retrospective chart review of patients with JS evaluated by movement disorder specialists.
-
Results
- Nine patients were included in the study. All patients had facial dysmorphism and ocular abnormalities, and 4 patients had dystonia. Ocular tilt reaction and alternate skew deviation (66%) were the most common ocular abnormalities. Horizontally aligned superior cerebellar peduncles were observed in all four patients with diffusion tensor imaging, with a lack of decussation in three. Exome sequencing performed in four patients revealed novel variants in the MKS1, CPLANE1, and PIBF1 genes.
-
Conclusion
- Facial dysmorphism, ocular abnormalities and classical imaging findings were observed in all patients with JS. Apart from ataxia, dystonia and myoclonus are other movement disorders observed in JS.
- This is a retrospective chart review of patients with JS evaluated by movement disorder specialists at National Institute of Mental Health and Neurosciences, India from 2014 to 2019. Patients with JS diagnosed based on the presence of MTS on imaging in the background of clinical features consistent with JS, such as developmental delay, apneic episodes and/or ataxia, were included in the study. The available demographics, clinical history, examination details, and investigations, including imaging and genetic data, were documented. In addition, patient videos were also reviewed. The data were expressed using descriptive statistics. The Institute Ethics Committee at the National Institute of Mental Health and Neurosciences granted an ethical clearance waiver owing to the retrospective nature of the study with de-identified data being extracted from files, and informed written consents were obtained for publication of recorded videos (No: NIMH/DO/DEAN [Basic Science]/2020-21).
MATERIALS & METHODS
- Nine patients (5 females, 55.5%) belonging to 8 families with a mean age of presentation of 10.3 ± 6.82 years (median: 12.5 years, range 10 months–24 years) were included. Of them, one patient (patient 3) was included in a previous report [7]. The demographic and clinical details are provided in Supplementary Table 1 (in the online-only Data Supplement).
- Clinical features
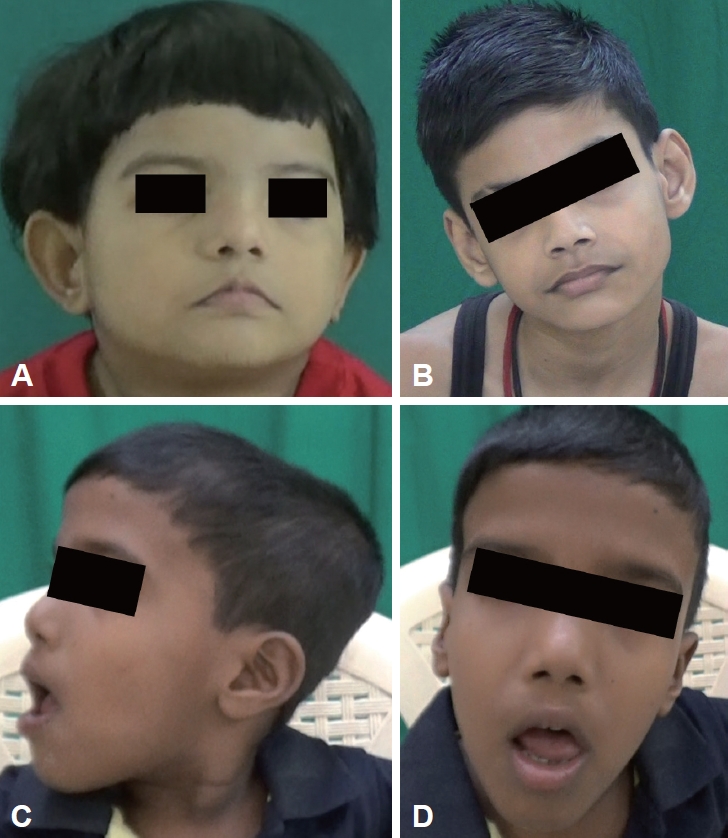
- Respiratory abnormalities such as apneic and hyperpneic episodes were seen in six patients in the neonatal period (Supplementary Table 1, Video 1 in the online-only Data Supplement). Developmental delay was noted in all patients, with severe developmental delay in 4 patients. One patient (patient 3) had generalized tonic clonic seizures (GTCSs) that were under control. All patients had facial dysmorphism, of which broad nasal bridges (n = 5) and low-set ears (n = 5) were most common (Supplementary Table 1 in the online-only Data Supplement, Figure 1). Head tilt was observed in 5 patients, and arachnodactyly and kyphosis were observed in 2 patients.
- Neurological examination
- Intellectual disability was noted in all patients, ranging from mild to moderate, with an intelligence quotient of less than 50 in 6 patients, while data were not available for the other 3 patients. Ocular abnormalities were seen in all patients (Supplementary Table 1 in the online-only Data Supplement), with congenital oculomotor apraxia (COMA) in 4 patients (Supplementary Video 2 in the online-only Data Supplement), ocular tilt reaction (OTR) in 6 patients (Supplementary Video 3 in the online-only Data Supplement) and alternate skew deviation (ASD) in 6 patients (Supplementary Video 4 in the online-only Data Supplement). Six patients had speech abnormalities, with hypernasality observed in 4 of these patients (Supplementary Video 5 in the online-only Data Supplement).
- Movement disorders
- A total of 4 patients had dystonia, of whom 2 patients had cervical dystonia (patients 4 and 9) (Supplementary Table 1 in the online-only Data Supplement), and 1 each had segmental dystonia involving the neck, trunk and upper limb (patient 6) (Supplementary Video 5 in the online-only Data Supplement) and generalized dystonia (patient 5). Multifocal myoclonus probably of reticular origin involving axial and proximal appendicular muscles present at rest and triggered by sound and action was noted in one patient who also had GTCS (patient 3). Four patients did not attain the gross motor milestone of walking, and gait ataxia was present in the other 5 patients.
- Investigations
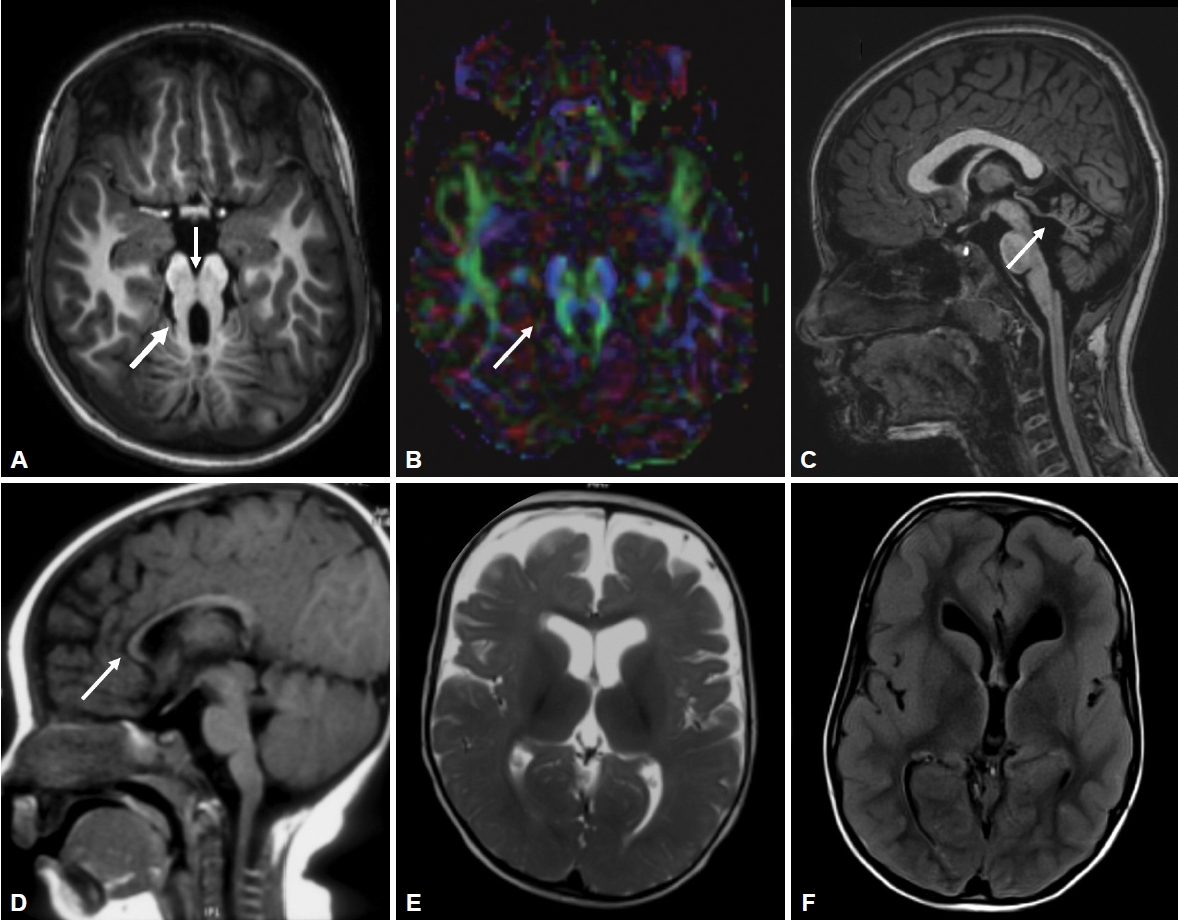
- All patients had MTS, deep interpeduncular fossa, vermian hypoplasia, superior cerebellar peduncle (SCP) thickening, fourth ventricular enlargement and batwing appearance of the fourth ventricle (Supplementary Table 2 in the online-only Data Supplement, Figure 2). Supratentorial abnormalities were observed in 3 patients (Figure 2). Diffusion tensor imaging (DTI) data were available for 4 patients, where horizontally oriented fibers were seen in all 4 and a lack of decussation was observed in 3 patients.
- Clinical exome sequencing was performed in 4 patients, and abnormalities were observed in CPLANE1 in patient 3, MKS1 in patients 5 and 6, and PIBF1 in patient 9. Five variants were identified in total, and all were novel (Supplementary Table 2 in the online-only Data Supplement). All were either pathogenic or likely pathogenic variants except for a variant of unknown significance (chr5:g.37247731A>G;p. Trp24Arg) found in patient 3. As the variant was in the compound heterozygous trans configuration with a pathogenic variant and in a patient with a typical phenotype, the variant was deduced as a disease-producing variation.
RESULTS
Imaging
Genetics
- JS is characterized by the classical features of hypotonia, apneic episodes in the newborn period followed by developmental delay, facial dysmorphism, ocular movement abnormalities and gradual development of ataxia [9]. The later mean age at presentation in our cohort compared to previous studies [10] might be due to referral bias. Additionally, in all our patients, apneic episodes disappeared by infancy, while the presence of prolonged apneic episodes is predictive of poor survival in JS [11]. Furthermore, none of our patients had multiorgan involvement, such as renal or cardiac issues.
- Facial dysmorphism is common, with features including a long face, prominent forehead, epicanthal folds, ptosis, arched eyebrows, broad nasal bridge, prognathism, everted lower lip, trapezoid mouth, and tongue protrusion [12]. Craniofacial abnormalities have been suggested to be associated with ciliary dysfunction [13]. However, they are variable and nonspecific and can overlap with other hindbrain syndromes, and genetic heterogeneity may add to the variability [12].
- Ocular abnormalities are one of the cardinal features of JS and include COMA, OTR, ASD, strabismus, nystagmus, ptosis, coloboma and retinopathy. COMA results from an inability to generate voluntary saccades compensated by head thrusts or frequent eye blinking. This irregularity in conjugate eye movements is a hallmark feature of JS. There can be frequent and periodic cyclic deviations of the eye, such as alternate gaze deviation, ASD, alternate torsional deviation, and wheel rolling torsional eye movements. Cyclic alternate torsional deviations, a type of OTR, are diagnostic of JS when seen in association with COMA [14,15]. It is characterized by spontaneous skew deviation, cyclotorsion of both eyes and paroxysmal head tilting, and it shares similarity with episodic apnea hyperpnea. These oculomotor abnormalities arise due to defects in cerebellar outflow pathways, nondecussation of the brainstem pathway and the loss of crossed inputs [15].
- Movement disorders apart from ataxia are rarely described in patients with JS. Ataxia in JS occurs due to vermian hypoplasia and nondecussation of SCP [10]. Cervical dystonia has been previously reported in a few case reports [5,6]. In our cohort, 5 patients had head tilt, which could be due to either cervical dystonia or oculomotor abnormalities. Even though the presence of additional appendicular dystonia in 2 patients may favor cervical dystonia, the absence of typical dystonic neck spasm or head tremor may suggest oculomotor abnormalities as the cause for head tilt. Myoclonus may also rarely occur and has been previously reported by our group [7].
- All patients with JS have cardinal magnetic resonance imaging features of MTS that occur due to deepened interpeduncular fossa, hypoplasia of the cerebellar vermis and thickened SCPs. Additionally, supratentorial findings can be associated, such as hippocampal malrotation, migration disorders, hydrocephalus, callosal dysgenesis and encephalocele (Figure 2). DTI in JS shows horizontal orientation of SCPs compared to vertically oriented SCPs in healthy subjects. The normal transverse decussation of SCPs at the level of inferior colliculi is visualized as a red dot on fractional anisotropy maps, and this is conspicuously absent in JS [16]. Of the 4 patients who had undergone DTI, all had horizontally oriented SCPs, while 3 had the absence of SCP decussation (Figure 2).
- JS is a genetically heterogeneous disorder with more than 30 genes identified thus far that are involved in producing proteins important for ciliary function [9]. There is variability in the genotype-phenotype correlations, and certain clinical features, such as orofacial digital abnormalities, renal abnormalities, retinal dystrophy, and hepatic abnormalities, may be attributable to a specific genetic mutation [17]. Patient 3 had abnormalities in the CPLANE1 gene with pure JS except for GTCS and multifocal myoclonus. Orofacial digital abnormalities have been described in JS with CPLANE1 mutations. However, up to two-thirds of cases can have a pure JS phenotype [18]. Mutations in the MKS1 gene may present as a pure JS phenotype or can be associated with retinal dystrophy, retinal coloboma, and renal and hepatic involvement [15,19]. Patient 6 with MKS1 gene abnormalities had retinal coloboma. Patient 9 with a pure JS phenotype had abnormalities in the PIBF1 gene, which can present either as pure JS or with retinal dystrophy, cystic kidney disease, liver fibrosis, and polydactyly [20]. These three genes play a role in ciliary function, explaining their involvement in JS.
- There are a few limitations to this study owing to the retrospective and cross-sectional nature. Systemic involvement in JS may develop over the course of the illness, and the absence of these symptoms at the time of evaluation may not imply the complete absence of these symptoms in the patient. Longitudinal studies are necessary to understand the temporal evaluation of symptoms in JS. A few patients seen at a very young age may not have the full spectrum of manifestations.
- To conclude, Joubert syndrome is a rare neurological disorder with a wide range of phenotypic features and significant genotypic variability. Facial dysmorphism and ocular abnormalities aid in suspecting Joubert syndrome, and apart from ataxia, the movement disorder spectrum of Joubert syndrome may include dystonia and myoclonus.
DISCUSSION
Supplementary Material
Video 1.
Video 3.
Video 4.
Video 5.
-
Conflicts of Interest
The authors have no financial conflicts of interest.
-
Funding Statement
None.
-
Author Contributions
Conceptualization: Bharath Kumar Surisetti, Vikram Venkappayya Holla, Shweta Prasad, Pramod Kumar Pal. Data curation: Bharath Kumar Surisetti, Vikram Venkappayya Holla, Shweta Prasad, Koti Neeraja. Formal analysis: Bharath Kumar Surisetti, Vikram Venkappayya Holla, Shweta Prasad. Investigation: Bharath Kumar Surisetti, Vikram Venkappayya Holla, Koti Neeraja. Methodology: Bharath Kumar Surisetti, Vikram Venkappayya Holla, Pramod Kumar Pal. Project administration: Nitish Kamble, Ravi Yadav, Pramod Kumar Pal. Resources: Vikram Venkappayya Holla, Nitish Kamble, Ravi Yadav, Pramod Kumar Pal. Supervision: Vikram Venkappayya Holla, Ravi Yadav, Pramod Kumar Pal. Visualization: Vikram Venkappayya Holla, Pramod Kumar Pal. Writing—original draft: Bharath Kumar Surisetti. Writing—review & editing: Vikram Venkappayya Holla, Shweta Prasad, Koti Neeraja, Nitish Kamble, Ravi Yadav, Pramod Kumar Pal.
Notes
- 1. Brancati F, Dallapiccola B, Valente EM. Joubert syndrome and related disorders. Orphanet J Rare Dis 2010;5:20.ArticlePubMedPMCPDF
- 2. Lee JE, Gleeson JG. Cilia in the nervous system: linking cilia function and neurodevelopmental disorders. Curr Opin Neurol 2011;24:98–105.ArticlePubMedPMC
- 3. Parisi MA. Clinical and molecular features of Joubert syndrome and related disorders. Am J Med Genet C Semin Med Genet 2009;151C:326–340.ArticlePubMedPMC
- 4. Joubert M, Eisenring JJ, Andermann F. Familial dysgenesis of the vermis: a syndrome of hyperventilation, abnormal eye movements and retardation. Neurology 1968;18:302–303.
- 5. Larson D, Kinsley L, Blackburn J, Mencacci N. Joubert’s syndrome with dystonic-ataxic tremor: a novel phenotypic variant. Neurology 2020;94:S2359.
- 6. Cinar S, Bilge S, Polat E, Akdemir O, Yildirim A. Cerebellar vermis hypoplasia in a case of Joubert syndrome associated with cervical dystonia: Mo-67. Movement Disorders 2009;24:S80.
- 7. Holla VV, Stezin A, Chaithra SP, Kamble N, Yadav R, Pal PK. Disabling myoclonus in a case of Joubert syndrome. Mov Disord Clin Pract 2020;7:456–458.ArticlePubMedPMCPDF
- 8. Maria BL, Quisling RG, Rosainz LC, Yachnis AT, Gitten J, Dede D, et al. Molar tooth sign in Joubert syndrome: clinical, radiologic, and pathologic significance. J Child Neurol 1999;14:368–376.ArticlePubMedPDF
- 9. Parisi M, Glass I. Joubert syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mirzaa G, et al., editors. GeneReviews (R) [Internet]; Seattle: University of Washington; c1993-2021. [accessed on 2021 May 20]. 2017;1-52. Available at: https://www.ncbi.nlm.nih.gov/books/NBK1325/.
- 10. Elhassanien AF, Alghaiaty HA. Joubert syndrome: clinical and radiological characteristics of nine patients. Ann Indian Acad Neurol 2013;16:239–244.ArticlePubMedPMC
- 11. Steinlin M, Schmid M, Landau K, Boltshauser E. Follow-up in children with Joubert syndrome. Neuropediatrics 1997;28:204–211.ArticlePubMed
- 12. Braddock SR, Henley KM, Maria BL. The face of Joubert syndrome: a study of dysmorphology and anthropometry. Am J Med Genet A 2007;143A:3235–3242.ArticlePubMed
- 13. Brugmann SA, Cordero DR, Helms JA. Craniofacial ciliopathies: a new classification for craniofacial disorders. Am J Med Genet A 2010;152A:2995–3006.ArticlePubMedPMC
- 14. Papanagnu E, Klaehn LD, Bang GM, Ghadban R, Mohney BG, Brodsky MC. Congenital ocular motor apraxia with wheel-rolling ocular torsiona neurodiagnostic phenotype of Joubert syndrome. J AAPOS 2014;18:404–407.ArticlePubMed
- 15. Wang SF, Kowal TJ, Ning K, Koo EB, Wu AY, Mahajan VB, et al. Review of ocular manifestations of Joubert syndrome. Genes (Basel) 2018;9:605.ArticlePubMedPMC
- 16. Poretti A, Boltshauser E, Loenneker T, Valente EM, Brancati F, Il’yasov K, et al. Diffusion tensor imaging in Joubert syndrome. AJNR Am J Neuroradiol 2007;28:1929–1933.ArticlePubMedPMC
- 17. Ben-Salem S, Al-Shamsi AM, Gleeson JG, Ali BR, Al-Gazali L. Mutation spectrum of Joubert syndrome and related disorders among Arabs. Hum Genome Var 2014;1:14020.ArticlePubMedPMCPDF
- 18. Wentzensen IM, Johnston JJ, Keppler-Noreuil K, Acrich K, David K, Johnson KD, et al. Exome sequencing identifies novel mutations in C5orf42 in patients with Joubert syndrome with oral-facial-digital anomalies. Hum Genome Var 2015;2:15045.ArticlePubMedPMCPDF
- 19. Romani M, Micalizzi A, Kraoua I, Dotti MT, Cavallin M, Sztriha L, et al. Mutations in B9D1 and MKS1 cause mild Joubert syndrome: expanding the genetic overlap with the lethal ciliopathy Meckel syndrome. Orphanet J Rare Dis 2014;9:72.ArticlePubMedPMCPDF
- 20. Hebbar M, Kanthi A, Shukla A, Bielas S, Girisha KM. A biallelic 36-bp insertion in PIBF1 is associated with Joubert syndrome. J Hum Genet 2018;63:935–939.ArticlePubMedPMCPDF
REFERENCES
Figure & Data
References
Citations

- Clinical and genetic characteristics of 36 children with Joubert syndrome
Yan Dong, Ke Zhang, He Yao, Tianming Jia, Jun Wang, Dengna Zhu, Falin Xu, Meiying Cheng, Shichao Zhao, Xiaoyi Shi
Frontiers in Pediatrics.2023;[Epub] CrossRef - CEP104 gene may involve in the pathogenesis of a new developmental disorder other than joubert syndrome
Reza Shervin Badv, Mojdeh Mahdiannasser, Maryam Rasoulinezhad, Laleh Habibi, Ali Rashidi-Nezhad
Molecular Biology Reports.2022; 49(8): 7231. CrossRef - Congenital Brain Malformations: An Integrated Diagnostic Approach
Bimal P. Chaudhari, Mai-Lan Ho
Seminars in Pediatric Neurology.2022; 42: 100973. CrossRef
Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite