Articles
- Page Path
- HOME > J Mov Disord > Volume 11(2); 2018 > Article
-
Original Article
Amantadine and the Risk of Dyskinesia in Patients with Early Parkinson’s Disease: An Open-Label, Pragmatic Trial -
Aryun Kim1, Young Eun Kim2, Ji Young Yun3, Han-Joon Kim1, Hui-Jun Yang4, Woong-Woo Lee5, Chae Won Shin6, Hyeyoung Park7, Yu Jin Jung8, Ahro Kim9, Yoon Kim1, Mihee Jang10, Beomseok Jeon1

-
Journal of Movement Disorders 2018;11(2):65-71.
DOI: https://doi.org/10.14802/jmd.18005
Published online: May 30, 2018
1Department of Neurology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
2Department of Neurology, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea
3Department of Neurology, Ewha Womans University School of Medicine, Ewha Womans University Mokdong Hospital, Seoul, Korea
4Department of Neurology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea
5Department of Neurology, Nowon Eulji Medical Center, Eulji University, Seoul, Korea
6Department of Neurology, Kyung Hee University Medical Center, Seoul, Korea
7Department of Neurology, Seoul Central Clinic, Seoul, Korea
8Department of Neurology, Daejeon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Daejeon, Korea
9Department of Neurology, Seoul St. Mary’s Hospital, Catholic University of Korea, Seoul, Korea
10Department of Neurology, Presbyterian Medical Center, Jeonju, Korea
- Corresponding author: Beomseok Jeon, MD, PhD, https://orcid.org/0000-0003-2491-3544 Department of Neurology, Seoul National University Hospital, Seoul National University College of Medicine, 101 Daehak-ro, Jongno-gu, Seoul 03080, Korea / Tel: +82-2-2072-2876 / Fax: +82-2-3672-7553 / E-mail: brain@snu.ac.kr
Copyright © 2018 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objective
- We examined whether amantadine can prevent the development of dyskinesia.
-
Methods
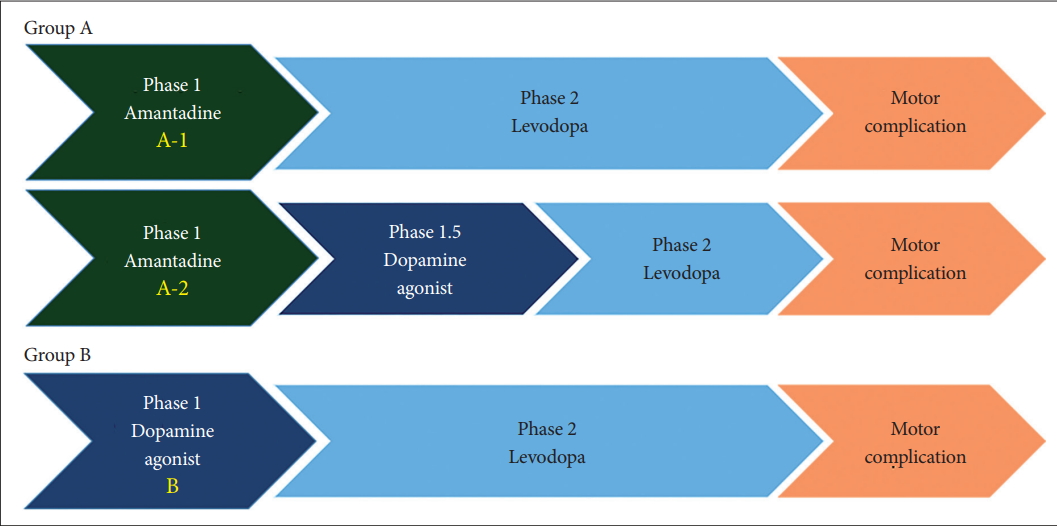
- Patients with drug-naïve Parkinson’s disease (PD), younger than 70 years of age and in the early stage of PD (Hoehn and Yahr scale < 3), were recruited from April 2011 to December 2014. The exclusion criteria included the previous use of antiparkinsonian medication, the presence of dyskinesia, significant psychological disorders, and previous history of a hypersensitivity reaction. Patients were consecutively assigned to one of 3 treatment groups in an open label fashion: Group A-1, amantadine first and then levodopa when needed; Group A-2, amantadine first, dopamine agonist when needed, and then levodopa; and Group B, dopamine agonist first and then levodopa when needed. The primary endpoint was the development of dyskinesia, which was analyzed by the Kaplan-Meier survival rate.
-
Results
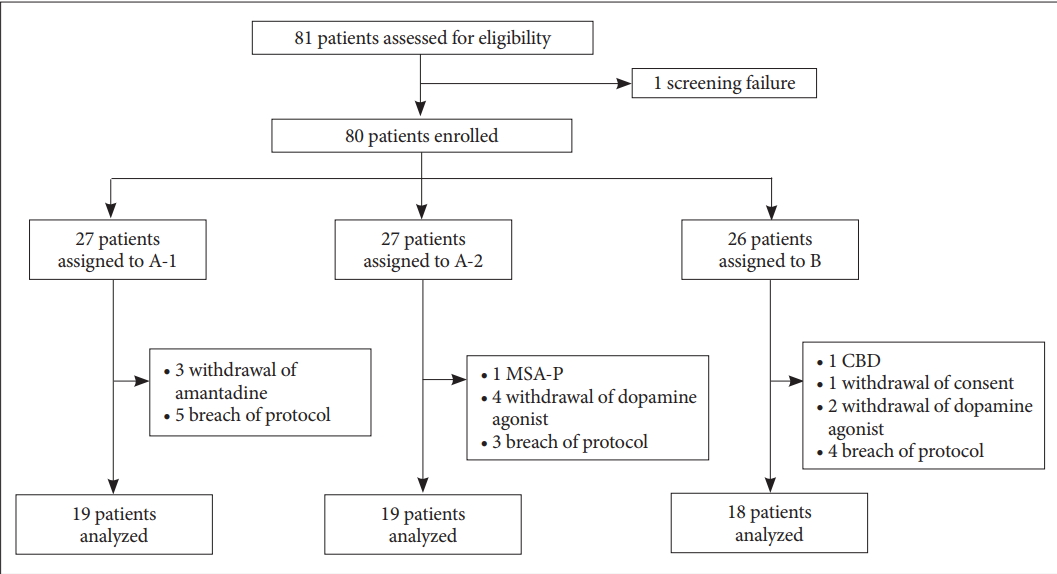
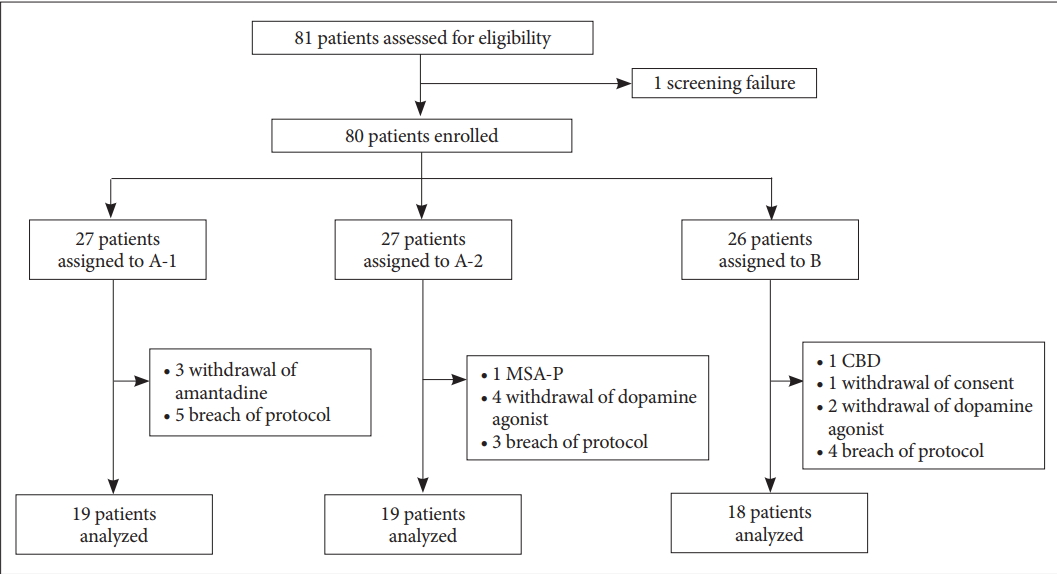
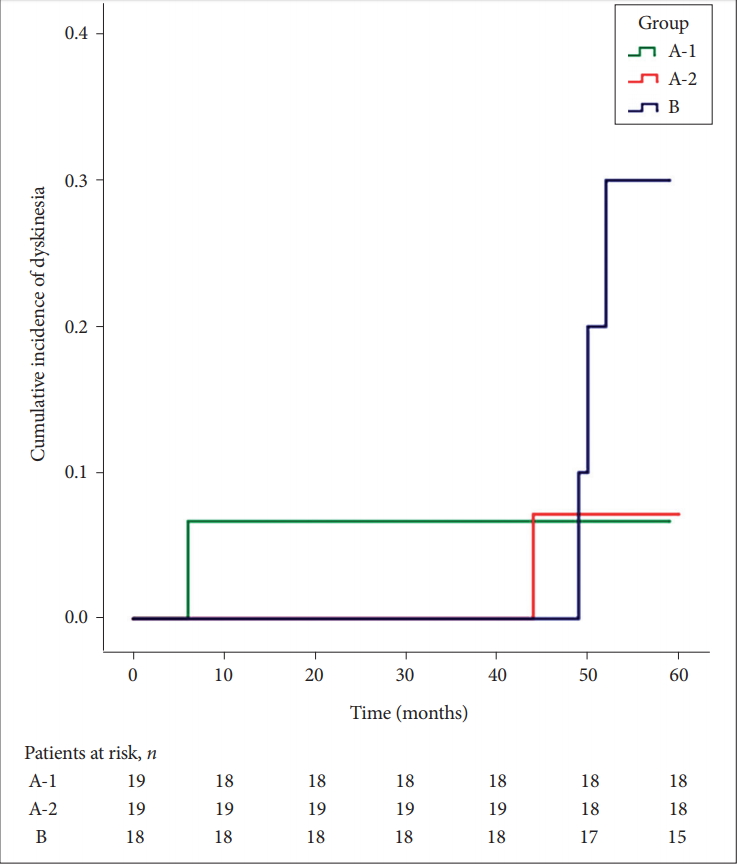
- A total of 80 patients were enrolled: Group A-1 (n = 27), Group A-2 (n = 27), and Group B (n = 26). Twenty-four patients were excluded from the analysis due to the following: withdrawal of amantadine or dopamine agonist (n = 9), alternative diagnosis (n = 2), withdrawal of consent (n = 1), and breach in the protocol (n = 12). After exclusion, 5 of the 56 (8.93%) patients developed dyskinesia. Patients in Group A-1 and A-2 tended to develop dyskinesia less often than those in Group B (cumulative survival rates of 0.933, 0.929, and 0.700 for A-1, A-2, and B, respectively; p = 0.453).
-
Conclusion
- Amantadine as an initial treatment may decrease the incidence of dyskinesia in patients with drug-naïve PD.
- Subjects
- Patients with PD who were newly diagnosed and were drug-naïve were recruited at the movement disorder clinic at Seoul National University Hospital. PD was diagnosed by movement disorder specialists based on the UK Parkinson’s Disease Society Brain Bank Criteria from April 2011 to December 2014. Patients younger than age 70 years and in the early stage of PD (Hoehn and Yahr stage < 3) at enrollment were included in the study. The exclusion criteria were as follows: previous use of antiparkinsonian medication, diagnosis of Parkinson plus syndrome, other significant psychological problems including major depression or dementia, previous history of hypersensitivity reaction to medication similar to the study drug, and possibility of pregnancy or breast-feeding. The participants were evaluated in the clinic by movement disorder specialists for 60 months on average at an interval of every 6 months. The analysis was conducted in July 2017.
- All patients in this study signed a written informed consent form according to the Declaration of Helsinki. The objectives and procedures of the study were approved by the Institutional Review Board (Seoul National University Hospital: reference number 1109-057-332). This trial is registered on ClinicalTrials.gov, identifier number NCT01338662.
- Study design
- ADAD was a single-center, prospective, open-label, parallel-group, and pragmatic trial. Patients were consecutively assigned to one of three treatment groups in an open-label manner. Patients in Group A-1 started the treatment with amantadine first, and then levodopa was added when needed. Amantadine was prescribed from 150 mg to 300 mg divided into 3 times a day. Patients in Group A-2 were prescribed amantadine first, dopamine agonist when needed, and then levodopa. Patients in Group B started with a dopamine agonist as an initial treatment, and then levodopa was added when needed. Amantadine was not prescribed in Group B unless dyskinesia developed. All patients were required to maintain amantadine and the dopamine agonist through the end of the study (Figure 1). When parkinsonian symptoms were not controlled well enough by the standard dose of amantadine or by the maximum tolerated dose of the dopamine agonists, investigators were allowed to add other drugs as needed. Selegiline, rasagiline, and entacapone were allowed to be added for optimal treatment at any time. On the other hand, any drug or treatment that could suppress LID, including deep brain stimulation and antipsychotics, was not allowed.
- Outcome measures
- The primary endpoint of the ADAD was the development of LID, including both peak-dose dyskinesia and diphasic dyskinesia. The secondary outcomes were as follows: 1) adverse events and adherence to each drug, 2) initial non-motor symptoms (NMS) as a predictor for the later development of dyskinesia, and 3) clinical characteristics and final doses of medication related to dyskinesia.
- The data on dyskinesia were collected in the clinic by interviews and neurologic examinations conducted by the movement disorder specialists. Neurologists interviewed the patients and evaluated the adverse events as well as the presence of dyskinesia. Baseline characteristics, including the age of onset, age at enrollment, sex, disease duration, Hoehn and Yahr stage, Unified Parkinson’s Disease Rating Scale (UPDRS), Mini Mental Status Examination, Frontal Assessment Battery, and initial NMS including REM sleep behavior disorder (RBD), constipation, urinary frequency or incontinence, and orthostatic dizziness, were evaluated at the first visit. The final doses of medication were calculated at the point of the development of dyskinesia for the patients with dyskinesia and at the last visit during 60 months for those without dyskinesia.
- Statistics
- Kaplan-Meier survival analysis was used to analyze the development of LID. All analyses followed the per-protocol analysis, and the log-rank test was used to examine the difference between the development rates of dyskinesia in each treatment group. We also applied the Cox proportional hazards model with Firth’s correction to evaluate the difference in the development of LID between the treatment groups. Kruskal-Wallis analysis and the Mann-Whitney U test were used to analyze the difference in the baseline characteristics of each group for numerical data. The chi-squared test and independent t-test were used for the univariate analysis with p < 0.05 as the threshold for statistical significance. SPSS software version 21 (IBM Corp., Armonk, NY, USA) was used for the statistical analysis.
MATERIALS & METHODS
- Levodopa-induced dyskinesia in each group
- A total of 80 patients (34 men and 46 women) were enrolled: Group A-1 (n = 27), Group A-2 (n = 27), and Group B (n = 26). During the trial, patients presenting any of the following were excluded from the analysis: 1) withdrawal of amantadine and dopamine agonist (n = 9), 2) alternative diagnosis on the follow-up (n = 2; multiple system atrophy and corticobasal degeneration), 3) withdrawal of consent (n = 1), and 4) breach in the protocol (n = 12) (Figure 2). A breach in the protocol included the following: dopamine agonist given in Group A-1 (n = 5), levodopa given before dopamine agonist in Group A-2 (n = 1), and use of antiparkinsonian medication before the study, which was recognized after the enrollment (n = 6). After the exclusion, 56 patients were eligible for Kaplan-Meier survival analysis for the development of dyskinesia. The baseline characteristics of the patients were comparable across the 3 treatment groups (Table 1). Median follow-up duration was 30.0 months.
- A total of 5 (8.93%) patients developed dyskinesia during the observation. The results showed that amantadine may reduce the incidence of LID, but it was not statistically significant. Patients in Group A-1 and A-2 who were administered amantadine for the initial treatment tended to develop dyskinesia less than those in Group B (cumulative survival rate of 0.933, 0.929, and 0.700 for A-1, A-2, and B, respectively). The p value in the log-rank test was 0.453 between all 3 groups and 0.207 between Group A-2 and B (Figure 3). When the Cox proportional hazard model with Firth’s correction was applied, the same trend was observed (hazard ratio of Group A-2 and B vs. A-1; 0.54 and 1.58, 95% confidence interval 0.04–7.26 and 0.18–14.09, p = 0.645 and 0.682). All dyskinesia in this study developed after the addition of levodopa. There was no significant difference in the final dose of medication, which included amantadine, dopamine agonist, and levodopa, between the 3 treatment groups (Supplementary Table 1 in the online-only Data Supplement).
- Characteristics of patients with LID
- The patients with LID were prescribed a significantly higher total levodopa equivalent daily dose at the last follow-up (p = 0.039). When amantadine and dopamine agonist doses were subtracted, the rest of the levodopa equivalent dose was also higher in patients with LID (p = 0.020). Other characteristics, including sex, age of onset, disease duration at enrollment, UPDRS, and clinical subtype of PD were not different between the patients with LID and those without LID (Supplementary Table 2 in the online-only Data Supplement). NMS at baseline, including RBD, constipation, urinary frequency or incontinence, and orthostatic dizziness, were not different between the patients with and without LID (chi-squared test, p = 0.554, 1.000, 1.000, and 1.000, respectively).
- Adverse events and adherence to the medication
- The adverse events of each drug are shown in Supplementary Table 3 (in the online-only Data Supplement). The most common adverse events were livedo reticularis for amantadine and dizziness for the dopamine agonist. Some of the adverse events were severe enough to stop the medication: 2 patients with livedo reticularis on amantadine, 3 patients with dizziness on the dopamine agonists, and 1 patient each with hypersomnia, edema, and impulse control disorder on the dopamine agonists.
RESULTS
- This study showed that amantadine as an initial treatment in patients with de novo PD tends to reduce the incidence of LID. In addition, the use of amantadine followed by dopamine agonist tends to lower the incidence of LID compared to dopamine agonist alone.
- The antidyskinetic effect of amantadine is considered to be related to the antagonistic activity at the N-methyl-D-aspartate (NMDA) receptor. The glutamatergic signaling from the cortex to the striatum goes through adaptive changes after chronic treatment with levodopa, resulting in an aberrant functioning of the NMDA receptors at the striatal medium spiny neuron dendritic spines [14]. The abnormal glutamatergic transmission in motor areas following levodopa administration in dyskinetic patients was also shown in one in vivo study [15]. In addition, trafficking and localization of NMDA receptor regulatory subunits are altered at the postsynaptic membrane in experimental models of LID [16]. Amantadine binds to the NMDA receptors and shows an inhibitory action, mainly through stabilization of closed states of the channel [17].
- Use of amantadine at an early stage of PD can avoid a direct effect on postsynaptic dopaminergic receptors so that pulsatile stimulation could be reduced when compared to using levodopa as an initial treatment. Development of LID is closely associated with both dopaminergic denervation and chronic pulsatile stimulation of dopamine receptors with levodopa [14,18]. The antiparkinsonian efficacy of amantadine was estimated as “likely efficacious” for symptomatic monotherapy and adjunct therapy by expert opinion, although its effect on postsynaptic dopamine receptors is not clear [19]. In addition, there were no serious adverse events. Livedo reticularis, the most common adverse event in the current study, is not life threatening and is reversible when stopped [20]. Amantadine can be safely used as an initial treatment for PD.
- In the current study, only 5 of 56 patients (8.93%) developed dyskinesia during a median follow-up period of 30.0 months, which is much lower compared to the previous study [1]. Because all the patients in the current study were educated about dyskinesia at the study enrollment, they were more aware of the risk of LID and tended to avoid increasing medication. They preferred to stay with parkinsonism in a tolerable range than taking more medication, which may explain the low incidence of dyskinesia in this study. In addition, there may have been undetected dyskinesia during the interviews and neurologic examinations because LID is a fluctuating symptom [8]. When compared with the patients without dyskinesia, the dyskinetic patients were taking more levodopa at the last follow-up, which is consistent with previous studies [4].
- In previous studies, the antidyskinetic effect of amantadine was examined by targeting patients who were already dyskinetic and evaluated for a short duration or in a wash-out manner. The antidyskinetic effect of amantadine was assessed for the first time during an acute intravenous levodopa infusion in a small placebo-controlled study [12]. Amantadine reduced peak-dose dyskinesia depending on its plasma level, and the effect was sustained for 1 year in the same patients [12,13]. In another placebocontrolled randomized trial with advanced PD patients, there was a reduction by 45% in the total dyskinesia scores after 15 days of amantadine treatment and rebound dyskinesia in 11 patients after withdrawal of amantadine [21]. Wolf et al. [22] reported that the withdrawal of amantadine in patients who had been taking amantadine more than 1 year worsened their dyskinesia when observed after 3 weeks. In a more recent randomized placebo-controlled study, wash-out of amantadine significantly worsened the dyskinesia without a significant effect on motor parkinsonism during the 3-month observation [9]. ADS-5102, an extended-release amantadine, also showed an antidyskinetic effect in patients with LID [11]. It is noteworthy that our findings targeted drug-naïve patients with PD, with a prospective design as well as a longer observation period.
- The current study included a relatively small number of patients, and the age of onset of the participants was younger compared to the epidemiological studies, such that our results may not represent all patients with PD [23,24]. Discontinuation of amantadine for a week every year was planned but was not done due to a lack of resources.
- To summarize, amantadine as an initial treatment may prevent the development of LID in the later course of PD. Amantadine can be considered the initial treatment in patients with de novo PD, even before starting dopamine agonists.
DISCUSSION
Supplementary Materials
-
Conflicts of Interest
B Jeon received a research grant from Kunil Pharmaceuticals, Peptron, Novartis Korea, Ipsen Korea, Samil Pharmaceuticals, Abbvie Korea, and Lundbeck Korea. He is a medical advisor to Bukwang Pharmaceuticals.
Notes
- The authors would like to thank Joongyub Lee (Biomedical Research Institution, Seoul National University Hospital), who provided the team with expert opinion on the statistical methods used in the project. This study was supported in part by the Seoul National University Hospital Grant (0620120560). We would also like to acknowledge the generous support of the Sinyang Cultural Foundation and Mr. Byung-Suk Park.
Acknowledgments
| All patients (n = 56) | A-1 (n = 19) | A-2 (n = 19) | B (n = 18) | p value | |
|---|---|---|---|---|---|
| Men | 26 (46.4) | 11 (57.9) | 6 (31.6) | 9 (50.0) | 0.249 |
| Age (yr) | 55.27 ± 8.69 | 54.95 ± 6.52 | 55.68 ± 9.22 | 55.17 ± 10.44 | 0.978 |
| Age of onset (yr) | 53.57 ± 9.41 | 53.32 ± 6.94 | 54.00 ± 9.76 | 53.39 ± 11.58 | 0.985 |
| PD duration (yr) | 1.71 ± 1.96 | 1.63 ± 1.83 | 1.68 ± 1.77 | 1.83 ± 2.36 | 0.955 |
| H&Y | 1.63 ± 0.54 | 1.47 ± 0.51 | 1.63 ± 0.50 | 1.81 ± 0.57 | 0.175 |
| UPDRS I | 1.48 ± 1.72 | 1.69 ± 1.67 | 1.33 ± 2.06 | 1.41 ± 1.42 | 0.521 |
| UPDRS II | 5.17 ± 4.00 | 5.44 ± 4.59 | 5.17 ± 4.60 | 4.88 ± 2.64 | 0.920 |
| UPDRS III | 19.29 ± 8.01 | 19.62 ± 9.12 | 17.85 ± 6.37 | 20.70 ± 8.70 | 0.727 |
| Tremor-dominant subtype* | 27 (54.0) | 9 (52.9) | 11 (55.0) | 7 (50.0) | 0.982 |
| MMSE | 27.10 ± 4.60 | 27.36 ± 1.91 | 25.93 ± 7.66 | 28.00 ± 1.56 | 0.744 |
| FAB | 15.71 ± 2.64 | 16.14 ± 1.23 | 15.00 ± 4.11 | 16.00 ± 1.62 | 0.959 |
Data are expressed as n (%) or mean ± standard deviation.
* calculations of the percentage of patients with the tremor-dominant subtype and the chi-squared test were performed after excluding 6 patients with incomplete evaluations. PD: Parkinson’s disease, H&Y: Hoehn and Yahr stage, UPDRS: Unified Parkinson’s Disease Rating Scale, MMSE: Mini-Mental Status Examination, FAB: Frontal Assessment Battery.
- 1. Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 2001;16:448–458.ArticlePubMed
- 2. Lyons KE, Hubble JP, Tröster AI, Pahwa R, Koller WC. Gender differences in Parkinson’s disease. Clin Neuropharmacol 1998;21:118–121.PubMed
- 3. Ku S, Glass GA. Age of Parkinson’s disease onset as a predictor for the development of dyskinesia. Mov Disord 2010;25:1177–1182.ArticlePubMed
- 4. Calabresi P, Di Filippo M, Ghiglieri V, Tambasco N, Picconi B. Levodopa-induced dyskinesias in patients with Parkinson’s disease: filling the bench-to-bedside gap. Lancet Neurol 2010;9:1106–1117.ArticlePubMed
- 5. Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE. A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. N Engl J Med 2000;342:1484–1491.ArticlePubMed
- 6. Noyes K, Dick AW, Holloway RG; Parkinson Study Group. Pramipexole v. levodopa as initial treatment for Parkinson’s disease: a randomized clinical-economic trial. Med Decis Making 2004;24:472–485.ArticlePubMed
- 7. Bracco F, Battaglia A, Chouza C, Dupont E, Gershanik O, Marti Masso JF, et al. The long-acting dopamine receptor agonist cabergoline in early Parkinson’s disease: final results of a 5-year, double-blind, levodopa-controlled study. CNS Drugs 2004;18:733–746.ArticlePubMed
- 8. Pilleri M, Antonini A. Therapeutic strategies to prevent and manage dyskinesias in Parkinson’s disease. Expert Opin Drug Saf 2015;14:281–294.ArticlePubMed
- 9. Ory-Magne F, Corvol JC, Azulay JP, Bonnet AM, Brefel-Courbon C, Damier P, et al. Withdrawing amantadine in dyskinetic patients with Parkinson disease: the AMANDYSK trial. Neurology 2014;82:300–307.ArticlePubMed
- 10. Duty S. Targeting glutamate receptors to tackle the pathogenesis, clinical symptoms and levodopa-induced dyskinesia associated with Parkinson’s disease. CNS Drugs 2012;26:1017–1032.ArticlePubMedPDF
- 11. Pahwa R, Tanner CM, Hauser RA, Isaacson SH, Nausieda PA, Truong DD, et al. ADS-5102 (amantadine) extendedrelease capsules for levodopa-induced dyskinesia in Parkinson disease (EASE LID Study): A Randomized Clinical Trial. JAMA Neurol 2017;74:941–949.ArticlePubMedPMC
- 12. Verhagen Metman L, Del Dotto P, van den Munckhof P, Fang J, Mouradian MM, Chase TN. Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson’s disease. Neurology 1998;50:1323–1326.ArticlePubMed
- 13. Metman LV, Del Dotto P, LePoole K, Konitsiotis S, Fang J, Chase TN. Amantadine for levodopa-induced dyskinesias: a 1-year follow-up study. Arch Neurol 1999;56:1383–1386.ArticlePubMed
- 14. Bastide MF, Meissner WG, Picconi B, Fasano S, Fernagut PO, Feyder M, et al. Pathophysiology of L-dopa-induced motor and non-motor complications in Parkinson’s disease. Prog Neurobiol 2015;132:96–168.ArticlePubMed
- 15. Ahmed I, Bose SK, Pavese N, Ramlackhansingh A, Turkheimer F, Hotton G, et al. Glutamate NMDA receptor dysregulation in Parkinson’s disease with dyskinesias. Brain 2011;134(Pt 4):979–986.ArticlePubMedPDF
- 16. Mellone M, Gardoni F. Modulation of NMDA receptor at the synapse: promising therapeutic interventions in disorders of the nervous system. Eur J Pharmacol 2013;719:75–83.ArticlePubMed
- 17. Blanpied TA, Clarke RJ, Johnson JW. Amantadine inhibits NMDA receptors by accelerating channel closure during channel block. J Neurosci 2005;25:3312–3322.ArticlePubMedPMC
- 18. Nadjar A, Gerfen CR, Bezard E. Priming for l-dopa-induced dyskinesia in Parkinson’s disease: a feature inherent to the treatment or the disease? Prog Neurobiol 2009;87:1–9.ArticlePubMed
- 19. Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA 2014;311:1670–1683.ArticlePubMed
- 20. Silver DE, Sahs AL. Livedo reticularis in Parkinson’s disease patients treated with amantadine hydrochloride. Neurology 1972;22:665–669.ArticlePubMed
- 21. Thomas A, Iacono D, Luciano AL, Armellino K, Di Iorio A, Onofrj M. Duration of amantadine benefit on dyskinesia of severe Parkinson’s disease. J Neurol Neurosurg Psychiatry 2004;75:141–143.PubMedPMC
- 22. Wolf E, Seppi K, Katzenschlager R, Hochschorner G, Ransmayr G, Schwingenschuh P, et al. Long-term antidyskinetic efficacy of amantadine in Parkinson’s disease. Mov Disord 2010;25:1357–1363.ArticlePubMed
- 23. Chen RC, Chang SF, Su CL, Chen TH, Yen MF, Wu HM, et al. Prevalence, incidence, and mortality of PD: a door-todoor survey in Ilan county, Taiwan. Neurology 2001;57:1679–1686.ArticlePubMed
- 24. Kusumi M, Nakashima K, Harada H, Nakayama H, Takahashi K. Epidemiology of Parkinson’s disease in Yonago City, Japan: comparison with a study carried out 12 years ago. Neuroepidemiology 1996;15:201–207.ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Investigation of the Long-Term Effects of Amantadine Use in Parkinson’s Disease
Sangmin Park, Jung Hwan Shin, Seung Ho Jeon, Chan Young Lee, Han-Joon Kim, Beomseok Jeon
Journal of Movement Disorders.2023; 16(2): 224. CrossRef - Polypharmazie bei der Behandlung von Parkinsonsymptomen: eine Nutzen-Risiko Abwägung
J. Bedarf, I. Csoti, H. Herbst, P. Urban, D. Woitalla, U. Wüllner
DGNeurologie.2023; 6(6): 504. CrossRef - Role of glutamate receptor complex in the organism. Ligands of NMDA receptors in neurodegenerative processes – a modern state of the problem
Vladimir D. Dergachev, Ekaterina E. Yakovleva, Eugenii R. Bychkov, Levon B. Piotrovskiy, Petr D. Shabanov
Reviews on Clinical Pharmacology and Drug Therapy.2022; 20(1): 17. CrossRef - Effect of glycine transporter 1 inhibition with bitopertin on parkinsonism and L-DOPA induced dyskinesia in the 6-OHDA-lesioned rat
Imane Frouni, Woojin Kang, Dominique Bédard, Sébastien Belliveau, Cynthia Kwan, Shadi Hadj-Youssef, Élodie Bourgeois-Cayer, Leanne Ohlund, Lekha Sleno, Adjia Hamadjida, Philippe Huot
European Journal of Pharmacology.2022; 929: 175090. CrossRef - Amantadine in the treatment of Parkinson’s disease. New opportunities in the context of COVID-19
E.A. Katunina
Zhurnal nevrologii i psikhiatrii im. S.S. Korsakova.2021; 121(4): 101. CrossRef - Current Knowledge on the Background, Pathophysiology and Treatment of Levodopa-Induced Dyskinesia—Literature Review
Michał Hutny, Jagoda Hofman, Aleksandra Klimkowicz-Mrowiec, Agnieszka Gorzkowska
Journal of Clinical Medicine.2021; 10(19): 4377. CrossRef - Neuroinflammation and blood–brain barrier disruption following traumatic brain injury: Pathophysiology and potential therapeutic targets
Suraj Sulhan, Kristopher A. Lyon, Lee A. Shapiro, Jason H. Huang
Journal of Neuroscience Research.2020; 98(1): 19. CrossRef - Emerging drugs for the treatment of L-DOPA-induced dyskinesia: an update
Sohaila AlShimemeri, Susan H Fox, Naomi P Visanji
Expert Opinion on Emerging Drugs.2020; 25(2): 131. CrossRef - Pharmacological Treatment of Early Motor Manifestations of Parkinson Disease (PD)
Michelle Ann C. Sy, Hubert H. Fernandez
Neurotherapeutics.2020; 17(4): 1331. CrossRef - Gut Microbiota Approach—A New Strategy to Treat Parkinson’s Disease
Jing Liu, Fei Xu, Zhiyan Nie, Lei Shao
Frontiers in Cellular and Infection Microbiology.2020;[Epub] CrossRef - Viewpoint: Developing drugs for levodopa‐induced dyskinesia in PD: Lessons learnt, what does the future hold?
Susan H. Fox, Jonathan M. Brotchie
European Journal of Neuroscience.2019; 49(3): 399. CrossRef - Polypharmacy in Parkinson’s disease: risks and benefits with little evidence
I. Csoti, H. Herbst, P. Urban, D. Woitalla, U. Wüllner
Journal of Neural Transmission.2019; 126(7): 871. CrossRef - Activation of mGlu2/3 receptors, a novel therapeutic approach to alleviate dyskinesia and psychosis in experimental parkinsonism
Imane Frouni, Adjia Hamadjida, Cynthia Kwan, Dominique Bédard, Vaidehi Nafade, Fleur Gaudette, Stephen G. Nuara, Jim C. Gourdon, Francis Beaudry, Philippe Huot
Neuropharmacology.2019; 158: 107725. CrossRef - Can therapeutic strategies prevent and manage dyskinesia in Parkinson’s disease? An update
Valentina Leta, Peter Jenner, K. Ray Chaudhuri, Angelo Antonini
Expert Opinion on Drug Safety.2019; 18(12): 1203. CrossRef
Comments on this article
- Figure
- Related articles
-
- Absence of Alpha-Synuclein Aggregation in Patients With Parkinson’s Disease Complicated by Sigmoid Volvulus
- Potential Link Between Cognition and Motor Reserve in Patients With Parkinson’s Disease
- Association Between Gait and Dysautonomia in Patients With De Novo Parkinson’s Disease: Forward Gait Versus Backward Gait
- Hand Movement-Induced Eyeblink Bursts in a Patient With Parkinson’s Disease
- Umami and Other Taste Perceptions in Patients With Parkinson’s Disease
 KMDS
KMDS
 E-submission
E-submission

 PubReader
PubReader ePub Link
ePub Link Cite
Cite