Articles
- Page Path
- HOME > J Mov Disord > Volume 15(2); 2022 > Article
-
Original Article
Umami and Other Taste Perceptions in Patients With Parkinson’s Disease -
Priya Jagota1
 , Nattida Chotechuang2
, Nattida Chotechuang2 , Chanawat Anan1
, Chanawat Anan1 , Teeraparp Kitjawijit3
, Teeraparp Kitjawijit3 , Chanchai Boonla4
, Chanchai Boonla4 , Roongroj Bhidayasiri1,5
, Roongroj Bhidayasiri1,5
-
Journal of Movement Disorders 2022;15(2):115-123.
DOI: https://doi.org/10.14802/jmd.21058
Published online: March 22, 2022
1Chulalongkorn Centre of Excellence for Parkinson’s Disease and Related Disorders, Department of Medicine, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Thai Red Cross Society, Bangkok, Thailand
2Department of Food Technology, Faculty of Science, Chulalongkorn University, Bangkok, Thailand
3Division of Neurology, Department of Medicine, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Thai Red Cross Society, Bangkok, Thailand
4Department of Biochemistry, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
5The Academy of Science, The Royal Society of Thailand, Bangkok, Thailand
- Corresponding author: Priya Jagota, MD, MSc Chulalongkorn Centre of Excellence for Parkinson’s Disease and Related Disorders, Department of Medicine, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Thai Red Cross Society, 1873 Rama 4 Road, Bangkok 10330, Thailand / Tel: +66-2-256-4000 ext. 71201-2 / Fax: +66-2-256-4000 ext. 70704 / E-mail: pja@chulapd.org
Copyright © 2022 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objective
- Studies of taste perceptions in Parkinson’s disease (PD) patients have been controversial, and none of these studies have assessed umami taste. This study aimed to assess umami, along with the other 4 taste functions in PD patients.
-
Methods
- Participants were tested for gustation using the modified filter paper disc method and olfaction using the modified Sniffin’ Stick-16 (mSS-16) test (only 14 culturally suitable items were used). A questionnaire evaluated patients’ subjective olfactory and gustatory dysfunction, taste preference, appetite, and food habits.
-
Results
- A total of 105 PD patients and 101 age- and sex-matched controls were included. The body mass index (BMI) of PD patients was lower than that of controls (PD = 22.62, controls = 23.86, p = 0.028). The mSS-16 score was 10.7 for controls and 6.4 for PD patients (p < 0.001) (normal ≥ 9). Taste recognition thresholds (RTs) for sweet, salty, sour, bitter and umami tastes were significantly higher in PD, indicating poorer gustation. All taste RTs correlated with each other, except for umami. Most patients were unaware of their dysfunction. Patients preferred sweet, salty and umami tastes more than the controls. Dysgeusia of different tastes in patients was differentially associated with poorer discrimination of tastes, an inability to identify the dish and adding extra seasoning to food. BMI and mSS-16 scores showed no correlation in either patients or controls.
-
Conclusion
- PD patients have dysgeusia for all five tastes, including umami, which affects their appetite and diet. Patients preferred sweet, salty and umami tastes. This information can help adjust patients’ diets to improve their nutritional status.
- This study was conducted in compliance with guidelines on human experimentation and approved by the Institutional Review Board of Faculty of Medicine, Chulalongkorn University (IRB no. 211/57). The study was conducted at King Chulalongkorn Memorial Hospital, Chulalongkorn University from 2015 to 2018. All the participants had a Thai Mental State Examination [20] (TMSE) score of at least 23 and provided informed consent. PD was diagnosed according to the United Kingdom Parkinson’s Disease Society Brain Bank (UKPDSBB) criteria [21]. Participants were excluded from the study if they had atypical parkinsonism, allergic rhinitis, upper respiratory tract infection, chronic sinusitis, chronic alcoholism, postoperative status, a history of severe traumatic brain injury or base of skull injury, a history of malignancy, chronic kidney disease, or hypothyroidism or a history of chemotherapy.
- Participants were asked about their perception of their sense of smell and taste. If they had abnormal perception, then they were further asked to identify whether the perception was decreased, absent or altered and whether the abnormalities were present only sometimes or all the time. Participants were asked to provide detailed information on food and taste preferences and appetite using a 25-item questionnaire. Participants’ weight and height were measured, and smell identification and gustatory tests were performed in both patients and controls.
- Smell identification test
- Patients and controls were tested with SS-16. We previously validated the SS-16 test kit in our patients with PD (unpublished data by T Kitjawijit and P Jagota, 2015). Only 14 odors were identified by more than 50% of the healthy controls and thus were considered culturally suitable. The cutoff score for olfactory dysfunction in patients with PD was identified as 9. For this study, we used only those 14 significantly relevant odors to test all the participants (turpentine and clove were not used), henceforth the test was designated the modified SS-16 (mSS-16).
- Gustatory (taste) identification test
- The filter paper disc method [22] (FPD) was modified by using cotton swabs to transfer the tastants instead of filter paper discs. In the FPD method, the filter paper discs are transferred to the tongue using forceps, where the examiner must ensure that the filter paper discs have been dropped onto the tongue and not moved elsewhere. Patients with PD experience rigidity and might have difficulty opening their mouths for a long time. Cotton swabs with the same bud size as filter paper discs (0.5 cm diameter) were used to ensure that an adequate amount of tastants transferred to overcome this problem.
- Solutions were prepared to test sweet, salty, sour, bitter, and umami tastes. A sucrose solution was used for sweet, sodium chloride for salty, tartaric acid for sour, quinine for bitter, and monosodium glutamate (MSG) for umami tastes. The concentrations of the solutions for sweet, sour, salty and bitter were prepared at 5 levels based on the FPD method [22]. For umami taste, 6 concentrations of MSG solutions were prepared using the method developed by Satoh-Kuriwada et al. [23] (Table 1).
- Participants had to refrain from smoking 1–2 hours before the test and refrain from eating, drinking, and chewing gum at least 30 minutes prior. They were tested by first applying the solution of a randomly selected taste with the lowest concentration (level 1), except for bitter, which was tested last to avoid unpleasantness. A cotton swab was dipped into the solution and then applied for 3 seconds onto the anterior (near the tip) part of the tongue. Then, the cotton swab was removed, and subjects were asked to swallow their saliva once to disperse the taste substance. Subsequently, the participants responded whether they had felt any taste and the name of the taste. If the taste was not identified, the next concentration of the same taste solution was tested using a new cotton swab until the taste was correctly identified or until the highest concentration of that taste solution was reached (level 5 for sweet, salty, sour, bitter and level 6 for umami). The concentration level at which the taste was identified was defined as the taste RT. If the taste was not identified, it was simply defined as “cannot be identified.” Then, the participants rinsed their mouth with water several times until no previous taste remained to avoid interference between tastes. The process was then repeated for other tastes in random order ending with bitter taste, as mentioned above. The same process was performed for both patients and controls. The total time for testing all the tastes in a participant was approximately 15 minutes.
- Statistical analysis
- The chi-square test and Fisher’s exact test were used for categorical data, the Mann–Whitney U test was used for ordinal data, and the independent t test was used for continuous data to test for significant differences between groups. Spearman’s rank correlation, point biserial correlation and Pearson’s correlation coefficients were calculated to study the correlations between variables. A p-value < 0.05 was considered statistically significant, except where a significant p-value was derived from the Benjamini-Hochberg procedure to adjust for multiple comparisons.
MATERIALS & METHODS
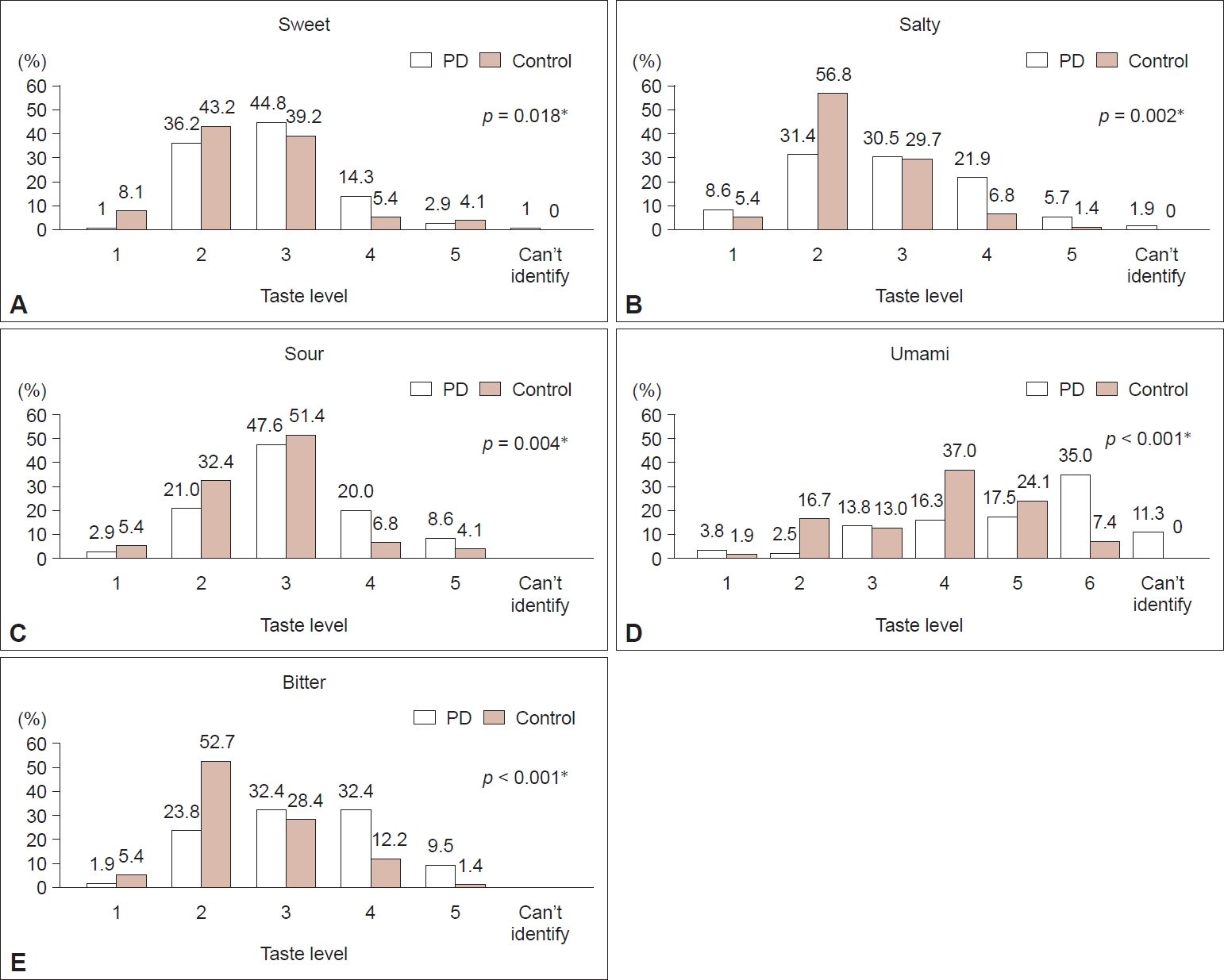
- One hundred five patients with PD and a total of 101 age- and sex-matched controls were included. Some of the controls did not answer some parts of the questionnaires; therefore, the number of controls is different for different parts. Demographic data are provided in Table 2. Weight and height were not different between patients with PD and controls, but the BMI was significantly higher in controls (PD = 22.62, controls = 23.86, p = 0.028), as well as smoking and drinking history (p = 0.009 and p = 0.006, respectively). The median Hoehn and Yahr (H&Y) stage was 2.5 (range 1–4). The prevalence of diabetes mellitus (DM) in patients and controls was 14.29% and 11.88% (p = 0.761), and hypertension (HT) was 25.71% and 37.62% (p = 0.091), respectively.
- Patients were more likely to perceive themselves as having abnormal smell and taste sensations than controls (p < 0.001 and p = 0.008, respectively) (Table 3). Most patients identified themselves as having decreased olfaction and gustation, with abnormalities present all the time in approximately 45% of the patients. Patients significantly preferred sweet (p = 0.001), salty (p = 0.004), and umami (p = 0.029) tastes compared to controls. The prevalence of dry mouth in both groups was not different (p = 0.461).
- Of the 25 items on the food and appetite questionnaire (Table 4), 3 items were significantly different between patients and controls after adjusting for multiple comparisons using the Benjamini-Hochberg procedure with a false discovery rate of 0.2. Patients felt that they had lost appetite (p = 0.001). Food taste was more important to the patients than controls (p = 0.001), and they had to add sugar or other sweet ingredients to their food more frequently than the controls (p = 0.012).
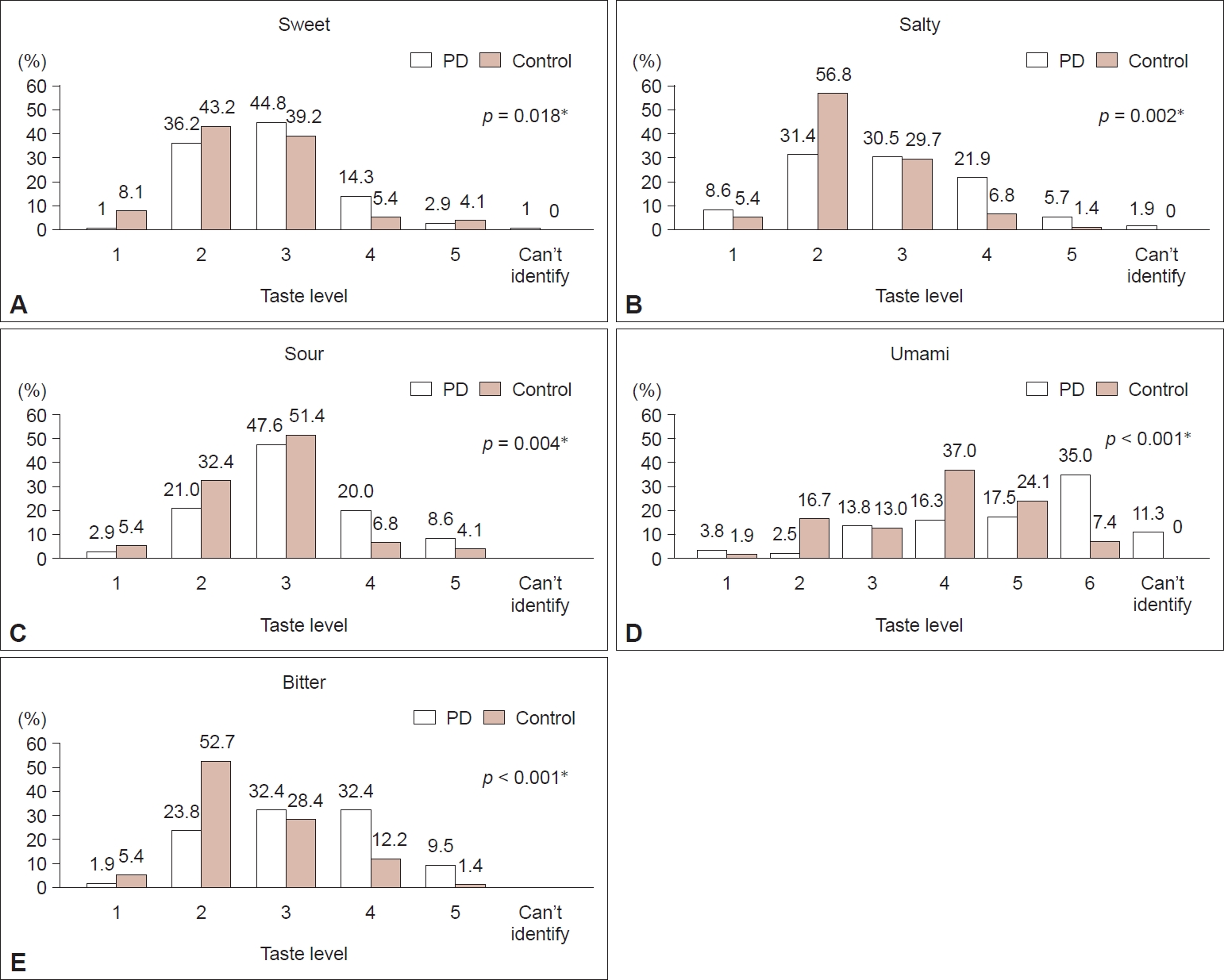
- The mean mSS-16 score (14 items) was 10.7 for controls and 6.4 for patients with PD (p < 0.001) (cutoff score for normal is 9) (Table 3). The gustatory RT test revealed that patients identified the correct tastes at a significantly higher concentration level for all tastes (Figure 1). The mode (highest frequency) of recognition for each taste in patients was level 3 for sweet, 2 for salty, 3 for sour, 3 and 4 for bitter and 6 for umami. In controls, it was level 2 for sweet, 2 for salty, 3 for sour, 2 for bitter and 4 for umami.
- Spearman’s correlation tests showed no correlation between the BMI and mSS-16 score in either patients or controls, or between the BMI and H&Y stage, and H&Y stage and mSS-16 in patients. In patients with PD, BMI exhibited a small but significant (p = 0.037, ρ = 0.204) correlation with the salty taste RT, and small correlations were observed between the H&Y stage with bitter (p = 0.030), and umami (p = 0.021) taste RTs (ρ = 0.212 and ρ = 0.258, respectively), indicating that a poorer salty taste sensation was associated with an increased BMI and poorer bitter and umami taste sensations were associated with more severe disease stages. In the control group, the bitter taste RT exhibited a small but significant inverse correlation with the mSS-16 score (p = 0.026, ρ = -0.258). Thus, a poorer bitter taste sensation was associated with a poorer olfactory score in controls.
- Spearman’s correlation analysis was conducted to study whether the five taste RTs had any correlations. All the tastes correlated positively with each other in both patients and controls, with moderate to large effect sizes (Table 5), except for umami and sweet RTs in patients with PD (p = 0.486), and umami and sweet (p = 0.059), umami and bitter (p = 0.055) and umami and sour (p = 0.369) RTs in controls.
- Regarding Pearson’s correlation coefficients between the 25 items on the food and appetite questionnaire (Table 4) and the five taste thresholds, the salt RT was negatively and mildly correlated with question 14 in patients with PD (p = 0.041, r = -0.20). The umami taste RT was also negatively and mildly correlated with question 14 (p = 0.019, r = -0.263) in the patients. Based on these results, higher salt and umami RTs, i.e., poorer salt and umami taste sensations, tend to be associated with a poorer ability of patients with PD to discriminate between different tastes of food. Umami was additionally mildly and negatively correlated with questions 15, 16 and 17 in patients (p = 0.026, r = -0.248; p = 0.012, r = -0.281; p = 0.026, r = -0.249, respectively). Therefore, a poorer umami taste sensation in patients with PD tends to be associated with a lower ability to discriminate the taste of food (e.g., sweet, salty, bitter, and sour), what dish it is and how spicy the food is. The sour taste RT was mildly correlated with question 5 in controls (p = 0.029, r = 0.253) and mildly and negatively correlated with question 20 in patients with PD (p = 0.029, r = -0.214). Based on this finding, a poorer sour taste sensation tends to be associated with weight loss in controls, and patients with PD are less likely to lose their appetite because of depression. The bitter taste RT was mildly correlated with question 6 in patients with PD (p = 0.029, r = 0.213). Thus, a poorer bitter taste sensation tends to be associated with the addition of extra seasoning to food by patients with PD. The sweet RT was not correlated with any of the questions in either patients or controls.
- The point biserial correlation analysis was conducted for the 25-item questionnaire and BMI and mSS-16. In patients with PD, BMI was negatively correlated with questions 5 and 19 (p = 0.029, rpb = -0.24 and p = 0.021, rpb = -0.25, respectively). BMI was positively correlated with question 25 (p = 0.027, rpb = 0.24). These results indicate that weight loss and financial difficulties tend to be associated with lower BMI values in patients with PD. Liking to eat what the patient or the patient’s family cooks rather than ready-to-eat food tends to be associated with higher BMI values. mSS-16 scores for both the patients and the controls and BMI of the controls were not correlated with any of the questions.
RESULTS
- Our study showed a significantly lower BMI in patients than in controls, consistent with previous studies [24,25], although their BMI was within the normal category. As reported in the literature, patients have impaired olfactory function. The present study showed that patients also have impaired gustatory function for all tastes, including umami, compared to controls. Previous studies have tested sweet, salty, sour, and bitter tastes [9-17]. They did not test umami taste. This study, to our knowledge, is the largest study testing taste sensation in patients with PD and is the only study that has tested umami taste sensation in patients with PD. Hypogeusia for all tastes was present, where patients identified all the tastes at higher concentration levels than the controls. One patient could not identify sweet taste, 2 could not identify salty taste, and 9 could not identify umami taste. All patients could identify sour and bitter tastes. Hence, the level of taste loss in patients may be unequal for different tastes. Cecchini et al. [26] showed that sour and salty taste identification was worse in patients with PD presenting a mild cognitive impairment and executive dysfunction. Another study in Asia by Kim et al. [15] found that female patients with PD had taste impairment, as identified by lower filter paper taste strip test (TST) scores, which tested sweet, sour, salty and bitter tastes (not umami). However, the impairment was attributable to a lower Mini-Mental State Examination (MMSE) score [15]. Therefore, differential taste loss might be the result of differential anatomical dysfunction. Regardless, all the patients in this study could still taste some of the tastants, although at higher concentrations.
- The loss of various tastes is correlated significantly and positively with each other. Hence, the loss of one taste will be associated with the loss of another taste, although they may be at different levels, as mentioned above. The exception for this is the umami taste. The umami taste threshold was not correlated with the sweet RT in patients or with the sweet, bitter or sour thresholds in controls. Moreover, more umami nonidentifiers were observed than any other taste. The level of the umami RT was also the highest (level 6 in patients and 4 in controls). It may explain the lack of a correlation. Previous studies have also reported a higher RT for umami than for other tastes in the elderly [27].
- Thai people consume more MSG per day than Japanese people (3.6 g/day vs. 1.2–1.7 g/day) [27]. Regular consumption of a larger amount of a tastant may increase its RT [28]. For the umami non-identifiers (as with other tastes) in this study, we do not know whether they have a RT that is higher than the maximum level in this study or whether they are ageusic for the taste. A study with a higher concentration of tastants may provide insights into this issue.
- Patients significantly perceived their abnormal olfaction and gustation. However, although the number of patients who perceived that their olfactory and gustatory functions were impaired was significantly higher than that of the controls, the majority of the patients still perceived them to be normal. This finding confirms previous reports that subjective taste and smell impairment identification is low [29,30].
- Based on the responses to the questionnaire, patients had to add sugar or other sweet ingredients to their food. Overall, the food and appetite questionnaire showed that dysgeusia in patients is associated with a poorer appetite, poorer ability to discriminate the taste of food and its spiciness, and a poorer ability to identify the dish. These limitations may have resulted in patients adding extra seasoning to their food. Losing weight and having financial difficulties were associated with a lower BMI, while eating home-cooked food was associated with a higher BMI in patients. On the other hand, the mSS-16 score was not associated with any of the questions. Their ageusia may have been attributed to their loss of appetite and rendered the taste of food more important to them. Hence, education on home-cooked, nutritious food with appropriate seasoning may be provided to patients and their caregivers to improve the patients’ appetite and nutrition and reduce their financial burden.
- In the present study, BMI did not correlate with olfactory function (mSS-16 score) in either patients or controls. It showed a small correlation with salty taste. The severity of the disease, as represented by H&Y staging, showed a small correlation with bitter and umami tastes but not with BMI and mSS-16 scores. Interestingly, in the controls, bitter taste exhibited a small inverse correlation with mSS-16 scores. Therefore, the poorer the olfactory function, the less participants can identify bitter taste. Correlation results vary from study to study. In contrast to this study, Roos et al. [11] found a small correlation between olfactory function and BMI but not gustatory function and BMI. Shah et al. [13] did not observe effects of age, disease severity or duration on gustation in patients with PD. In contrast, De Rosa et al. [10] identified a correlation between gustation and disease severity and stage. Kim et al. [15] found no significant correlations between the TST score and age, H&Y stage, disease duration, MMSE score, MoCA score, or smell test score, even when their data were analyzed separately according to sex.
- Taste preference in humans has developed since childhood. The body usually prefers particular tastes to obtain the nutrients it needs–sweet for energy (carbohydrates), salty for minerals and umami (savory) tastes for proteins [31]. A bitter taste represents toxic food, and sour represents acids, and thus these foods are usually avoided [31]. Patients in this study preferred sweet, salty, and umami tastes more than the controls. Increased energy and nutritional requirements from motor symptoms and malnutrition may explain these preferences [24,32]. Patients added significantly more sweet ingredients to their food. The sweet preference in humans is evident beginning in the prenatal period [33]. Some studies have shown that, unlike other tastes’ RT, sweet RT does not significantly increase with aging [30,34,35]. Meyers et al. [36] reported an increased sweet preference, sweet consumption, and ice cream preference in patients with PD compared to controls. However, in another study by Sienkiewicz-Jarosz et al. [37], pleasantness ratings of sucrose solutions did not differ between patients and controls. However, this study involved only 20 patients.
- Malnutrition is more prevalent in the later stages of PD [32]. As patients with a low BMI have a poorer survival prognosis [38], malnutrition prevention should be initiated early in the course of the disease to maintain a normal BMI. Knowledge of patients’ preferences for food taste and type can help relieve this issue. Flavor has sometimes been confused with taste. Olfaction, gustation, and mechanosensation of the tongue together send signals to the orbitofrontal cortex where the pleasantness or unpleasantness of food is perceived. This perception constitutes flavor. The smell or taste of the food along with the texture, sight, and anticipation of the food affect flavor. Therefore, studies aiming to improve nutrition or quality of life (QoL) related to food intake may need to consider factors other than olfaction and gustation.
- This study has a few limitations. First, the validation of SS-16 in patients with PD by our group is unpublished (by T Kitjawijit and P Jagota, 2015). Second, although the BMI of patients was lower than that of the controls, patients with PD included in the present study had a normal BMI. Hence, the result may be different in malnourished or low BMI patients. As the study showed impaired gustation in this group with an almost high BMI, the impairment may be more pronounced in the lower BMI group. Further studies will be needed to validate this assumption. We did not study the effects of medications on tastes, which is another limitation of this study, although a previous study has shown that the taste test score was not associated with the levodopa equivalent dose [9]. Some small correlations between some tastes and the H&Y stage and BMI were observed in patients. As stated above, correlation studies remain controversial. More extensive studies are needed to investigate this issue further.
DISCUSSION
- This study confirms olfactory and gustatory dysfunction for all tastes, including umami, in patients with PD. Most of the patients are unaware of them. They may unconsciously add extra seasoning or sweet ingredients to overcome taste dysfunction.
- Patients have a preference for sweet, salty, and umami tastes. Increased sweet consumption is evidenced by adding extra sweet ingredients to their food. This information may be of use when providing counseling or adjusting patients’ diets to treat or prevent malnutrition. Adjustments in the diet must counterbalance increased risks of DM, HT, and other acquired disorders in patients. The addition of artificial sweeteners, food enhancers and seasonings, an understanding of food combination types according to ethnicity, or other strategies may need to be used to improve the “flavor” of food and QoL of patients.
Conclusions
-
Conflicts of Interest
The authors have no financial conflicts of interest.
-
Funding Statement
This study was funded by the Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University, grant number RA57/112, Chulalongkorn Academic Advancement Fund into Its 2nd Century Project of Chulalongkorn University (2300042200), and Centre of Excellence Grant of Chulalongkorn University (GCE6100930004-1).
-
Author Contributions
Conceptualization: Priya Jagota, Nattida Chotechuang, Chanchai Boonla, Roongroj Bhidayasiri. Formal analysis: Priya Jagota, Nattida Chotechuang. Funding acquisition: Roongroj Bhidayasiri. Investigation: Teeraparp Kitjawijit. Methodology: Priya Jagota, Nattida Chotechuang, Chanchai Boonla, Roongroj Bhidayasiri. Project administration: Priya Jagota, Nattida Chotechuang, Chanawat Anan, Teeraparp Kitjawijit. Resources: Priya Jagota, Nattida Chotechuang, Chanawat Anan, Teeraparp Kitjawijit. Supervision: Chanchai Boonla, Roongroj Bhidayasiri. Writing—original draft: Priya Jagota. Writing—review & editing: all authors.
Notes
| Demographic data | PD (n = 105) | Control (n = 101) | p-value |
|---|---|---|---|
| Age (yr) | 63.3 ± 10.5 | 61.07 ± 11.1 | 0.141† |
| Sex | |||
| Male | 50 (47.6) | 41 (40.6) | 0.310‡ |
| Female | 55 (52.4) | 60 (59.4) | |
| Weight (kg) | 58.57 ± 12.9 | 60.66 ± 12.5 | 0.279† |
| Height (cm) | 160.48 ± 8.8 | 159.01 ± 8.8 | 0.276† |
| BMI | 22.62 ± 3.9 | 23.86 ± 4 | 0.028*† |
| Smoking | |||
| Never | 83 (79) | 82 (81.2) | 0.009*‡ |
| Current smokers | 2 (1.9) | 10 (9.9) | |
| Past smokers | 20 (19) | 9 (8.9) | |
| Alcohol drinking | |||
| Never | 73 (69.5) | 86 (85.1) | 0.006*‡ |
| Current drinkers | 10 (9.5) | 9 (8.9) | |
| Past drinkers | 22 (21) | 6 (5.9) | |
| Age at PD onset (yr) | 53.9 ± 12.3 | NA | |
| Current symptoms | |||
| Tremor | 61 (58.1) | NA | |
| Rigidity | 57 (54.3) | NA | |
| Bradykinesia | 73 (69.5) | NA | |
| Postural instability | 34 (32.4) | NA | |
| Gait problem | 50 (47.6) | NA | |
| Motor complications | |||
| Wearing off | 38 (36.2) | NA | |
| Dyskinesia | 24 (22.9) | NA | |
| H&Y§ | |||
| 1 | 1 (1) | NA | |
| 1.5 | 5 (4.8) | NA | |
| 2 | 20 (19) | NA | |
| 2.5 | 61 (58.1) | NA | |
| 3 | 16 (15.2) | NA | |
| 4 | 2 (1.9) | NA |
| Parameters | PD (n = 105) | Control (n = 101) | p-value |
|---|---|---|---|
| Mean modified Sniffin’ Stick-16 score (total 14 odors)∥ | 6.4 | 10.7 | < 0.001*† |
| Is your smell sensation normal? | |||
| Yes | 78 (74.3) | 61 (98.4) (n = 62) | < 0.001*‡ |
| No | 27 (25.7) | 1 (1.6) | |
| Olfaction abnormality | |||
| Decreased | 23 (85.2) | 1 (100) | |
| Absent | 2 (7.4) | 0 (0) | |
| Altered | 2 (7.4) | 0 (0) | |
| Persistence of olfaction abnormality | |||
| Present all the time | 12 (44.4) | 1 (100) | |
| Present sometimes | 15 (55.6) | 0 (0) | |
| Is your taste sensation normal? | |||
| Yes | 90 (85.7) | 60 (98.4) (n = 61) | 0.008*‡ |
| No | 15 (14.3) | 1 (1.6) | |
| Gustatory abnormality | |||
| Decreased | 13 (86.7) | 1 (100) | |
| Absent | 1 (6.7) | 0 (0) | |
| Altered | 1 (6.7) | 0 (0) | |
| Persistence of gustatory abnormality | |||
| Present all the time | 7 (46.7) | 1 (100) | |
| Present sometimes | 8 (53.3) | 0 (0) | |
| Preferred taste | |||
| Sweet | 54 (51.4) | 29 (28.7) | 0.001*‡ |
| Salty | 35 (33.3) | 16 (15.8) | 0.004*‡ |
| Sour | 36 (34.3) | 42 (41.6) | 0.280‡ |
| Bitter | 1 (1) | 2 (2) | 0.616§ |
| Umami | 6 (5.7) | 0 (0) | 0.029*§ |
| Dry mouth | 8 (7.6) | 8 (10.8) (n = 74) | 0.461‡ |
Values are presented as n (%) unless otherwise indicated. Some of the controls did not answer some parts of the questionnaires, therefore, the number of controls is different for different parts.
* p < 0.05;
† independent t test;
‡ chi-square test;
§ Fisher’s exact test;
∥ validation of the smell test in the Thai Parkinson’s disease (PD) population shows that only 14 items are useful for the test (turpentine and clove were omitted from the test).
| No. | Questions | PD (n = 105) | Control (n = 74) | p-value* |
|---|---|---|---|---|
| 1 | You feel that you eat less than before. | 36.2 | 23 | 0.059 |
| 2 | Do you feel that you experience the taste of food less than before? | 32.4 | 18.9 | 0.045 |
| 3 | You feel that your food is not as tasty as before. | 39 | 25.7 | 0.062 |
| 4 | You have lost your appetite. | 34.3 | 12.2 | 0.001† |
| 5 | You have lost weight. | 34.3 | 20.3 | 0.041 |
| 6 | You must add extra seasoning to every food. | 25.7 | 23 | 0.675 |
| 7 | You feel that your taste sensation has changed. | 26.7 | 13.5 | 0.034 |
| 8 | When eating food, you must add fish sauce, salt, soy sauce, or other salty ingredients. | 29.5 | 20.3 | 0.163 |
| 9 | When eating food, you must add lemon, vinegar, or other sour ingredients. | 26.7 | 31.1 | 0.519 |
| 10 | When eating food, you must add sugar or other sweet ingredients. | 29.5 | 13.5 | 0.012† |
| 11 | When eating food, you must add monosodium glutamate or other ingredients to make the food taste better. | 13.3 | 16.2 | 0.590 |
| 12 | When eating food, you must add chili. | 34.3 | 40.5 | 0.393 |
| 13 | The taste of food is very important to you. | 67.6 | 43.2 | 0.001† |
| 14 | You can discriminate between different tastes of food, e.g., salty, sweet, sour, and bitter. | 86.7 | 82.4 | 0.436 |
| 15 | When you start eating, you can determine the taste of the food. | 92.4 | 82.4 | 0.042 |
| 16 | When you start eating, you can identify what dish it is. | 88.6 | 81.1 | 0.161 |
| 17 | You can determine how spicy the food is. | 86.7 | 82.4 | 0.436 |
| 18 | You have oral health problems, such as tooth decay, broken teeth or oral ulcers. | 51.4 | 45.9 | 0.470 |
| 19 | Your financial difficulties limit your food choices. | 12.4 | 17.6 | 0.332 |
| 20 | Your depression causes a loss of appetite. | 18.1 | 10.8 | 0.180 |
| 21 | Your nausea or vomiting causes a loss of appetite. | 9.5 | 5.4 | 0.312 |
| 22 | You feel that your medication causes nausea and loss of appetite. | 9.5 | 12.2 | 0.573 |
| 23 | You feel that you want to eat more when you add extra seasoning. | 41.9 | 32.4 | 0.199 |
| 24 | You cook yourself. | 61.9 | 59.5 | 0.741 |
| 25 | You like to eat what you or your family cook rather than ready-to-eat food. | 74.3 | 78.4 | 0.528 |
| Combination |
PD |
Control |
||
|---|---|---|---|---|
| p-value* | p† | p-value* | p† | |
| Sweet recognition threshold & salt recognition threshold | < 0.001* | 0.38 | 0.023* | 0.31 |
| Sweet recognition threshold & bitter recognition threshold | < 0.001* | 0.38 | < 0.001* | 0.70 |
| Sweet recognition threshold & sour recognition threshold | 0.011* | 0.28 | < 0.001* | 0.45 |
| Sweet recognition threshold & umami recognition threshold | 0.486 | 0.08 | 0.059 | 0.26 |
| Salt recognition threshold & bitter recognition threshold | 0.002* | 0.34 | 0.002* | 0.42 |
| Salt recognition threshold & sour recognition threshold | < 0.001* | 0.38 | 0.024* | 0.31 |
| Salt recognition threshold & umami recognition threshold | 0.007* | 0.30 | 0.016* | 0.33 |
| Bitter recognition threshold & sour recognition threshold | < 0.001* | 0.36 | 0.009* | 0.35 |
| Bitter recognition threshold & umami recognition threshold | 0.026* | 0.25 | 0.055 | 0.26 |
| Sour recognition threshold & umami recognition threshold | < 0.001* | 0.37 | 0.369 | -0.12 |
- 1. Pont-Sunyer C, Hotter A, Gaig C, Seppi K, Compta Y, Katzenschlager R, et al. The onset of nonmotor symptoms in Parkinson’s disease (the ONSET PD study). Mov Disord 2015;30:229–237.ArticlePubMed
- 2. Hawkes CH, Shephard BC, Daniel SE. Olfactory dysfunction in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1997;62:436–446.ArticlePubMedPMC
- 3. Oppo V, Melis M, Melis M, Tomassini Barbarossa I, Cossu G. “Smelling and tasting” Parkinson’s disease: using senses to improve the knowledge of the disease. Front Aging Neurosci 2020;12:43.ArticlePubMedPMC
- 4. Doty RL. Measurement of chemosensory function. World J Otorhinolaryngol Head Neck Surg 2018;4:11–28.ArticlePubMedPMC
- 5. Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania smell identification test: a rapid quantitative olfactory function test for the clinic. Laryngoscope 1984;94:176–178.ArticlePubMed
- 6. Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 1997;22:39–52.ArticlePubMed
- 7. Cecchini MP, Fasano A, Boschi F, Osculati F, Tinazzi M. Taste in Parkinson’s disease. J Neurol 2015;262:806–813.ArticlePubMed
- 8. Khan AM, Ali S, Jameela RV, Muhamood M, Haqh MF. Impact of fungiform papillae count on taste perception and different methods of taste assessment and their clinical applications: a comprehensive review. Sultan Qaboos Univ Med J 2019;19:e184–e191.PubMedPMC
- 9. Doty RL, Nsoesie MT, Chung I, Osman A, Pawasarat I, Caulfield J, et al. Taste function in early stage treated and untreated Parkinson’s disease. J Neurol 2015;262:547–557.ArticlePubMed
- 10. De Rosa A, Nettore IC, Cantone E, Maione L, Desiderio S, Peluso S, et al. The flavor test is a sensitive tool in identifying the flavor sensorineural dysfunction in Parkinson’s disease. Neurol Sci 2019;40:1351–1356.ArticlePubMed
- 11. Roos DS, Oranje OJM, Freriksen AFD, Berendse HW, Boesveldt S. Flavor perception and the risk of malnutrition in patients with Parkinson’s disease. J Neural Transm (Vienna) 2018;125:925–930.ArticlePubMedPMC
- 12. Sienkiewicz-Jarosz H, Scinska A, Kuran W, Ryglewicz D, Rogowski A, Wrobel E, et al. Taste responses in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 2005;76:40–46.ArticlePubMedPMC
- 13. Shah M, Deeb J, Fernando M, Noyce A, Visentin E, Findley LJ, et al. Abnormality of taste and smell in Parkinson’s disease. Parkinsonism Relat Disord 2009;15:232–237.ArticlePubMed
- 14. Deeb J, Shah M, Muhammed N, Gunasekera R, Gannon K, Findley LJ, et al. A basic smell test is as sensitive as a dopamine transporter scan: comparison of olfaction, taste and DaTSCAN in the diagnosis of Parkinson’s disease. QJM 2010;103:941–952.ArticlePubMed
- 15. Kim HJ, Jeon BS, Lee JY, Cho YJ, Hong KS, Cho JY. Taste function in patients with Parkinson disease. J Neurol 2011;258:1076–1079.ArticlePubMed
- 16. Cecchini MP, Osculati F, Ottaviani S, Boschi F, Fasano A, Tinazzi M. Taste performance in Parkinson’s disease. J Neural Transm (Vienna) 2014;121:119–122.ArticlePubMed
- 17. Ricatti MJ, Ottaviani S, Boschi F, Fasano A, Tinazzi M, Cecchini MP. A prospective evaluation of taste in Parkinson’s disease. J Neural Transm (Vienna) 2017;124:347–352.ArticlePubMed
- 18. Ninomiya K. Science of umami taste: adaptation to gastronomic culture. Flavour 2015;4:13.Article
- 19. Kurihara K. Umami the fifth basic taste: history of studies on receptor mechanisms and role as a food flavor. Biomed Res Int 2015;2015:189402.ArticlePubMedPMC
- 20. Train the Brain Forum Committee (Thailand). Thai mental state examination (TMSE). Siriraj Hospital Gazette 1993;45:359–374.
- 21. Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 1988;51:745–752.ArticlePubMedPMC
- 22. Nagai A, Kubota M, Katayama Y, Kojima C. Evaluation of taste acuity by the filter-paper disc in Japanese young women: the relationship with micronutrients status. Asia Pac J Clin Nutr 2012;21:406–410.PubMed
- 23. Satoh-Kuriwada S, Kawai M, Iikubo M, Sekine-Hayakawa Y, Shoji N, Uneyama H, et al. Development of an umami taste sensitivity test and its clinical use. PLoS One 2014;9:e95177. ArticlePubMedPMC
- 24. Bachmann CG, Trenkwalder C. Body weight in patients with Parkinson’s disease. Mov Disord 2006;21:1824–1830.ArticlePubMed
- 25. van der Marck MA, Dicke HC, Uc EY, Kentin ZH, Borm GF, Bloem BR, et al. Body mass index in Parkinson’s disease: a meta-analysis. Parkinsonism Relat Disord 2012;18:263–267.ArticlePubMed
- 26. Cecchini MP, Federico A, Zanini A, Mantovani E, Masala C, Tinazzi M, et al. Olfaction and taste in Parkinson’s disease: the association with mild cognitive impairment and the single cognitive domain dysfunction. J Neural Transm (Vienna) 2019;126:585–595.ArticlePubMed
- 27. Trachootham D, Satoh-Kuriwada S, Lam-Ubol A, Promkam C, Chotechuang N, Sasano T, et al. Differences in taste perception and spicy preference: a Thai–Japanese cross-cultural study. Chem Senses 2017;43:65–74.ArticlePubMed
- 28. Noel CA, Finlayson G, Dando R. Prolonged exposure to monosodium glutamate in healthy young adults decreases perceived umami taste and diminishes appetite for savory foods. J Nutr 2018;148:980–988.ArticlePubMed
- 29. Soter A, Kim J, Jackman A, Tourbier I, Kaul A, Doty RL. Accuracy of self-report in detecting taste dysfunction. Laryngoscope 2008;118:611–617.ArticlePubMed
- 30. Welge-Lüssen A, Dörig P, Wolfensberger M, Krone F, Hummel T. A study about the frequency of taste disorders. J Neurol 2011;258:386–392.ArticlePubMed
- 31. Breslin PA. An evolutionary perspective on food and human taste. Curr Biol 2013;23:R409–R418.ArticlePubMedPMC
- 32. Budrewicz S, Zmarzły A, Rączka D, Szczepańska A, Koziorowska-Gawron E, Słotwiński K, et al. Clinical and nutritional correlations in Parkinson’s disease: preliminary report. Adv Clin Exp Med 2019;28:193–198.ArticlePubMed
- 33. Maone TR, Mattes RD, Bernbaum JC, Beauchamp GK. A new method for delivering a taste without fluids to preterm and term infants. Dev Psychobiol 1990;23:179–191.ArticlePubMed
- 34. Yamauchi Y, Endo S, Yoshimura I. A new whole-mouth gustatory test procedure. II. Effects of aging, gender and smoking. Acta Otolaryngol Suppl 2002;546:49–59.Article
- 35. Weiffenbach JM, Baum BJ, Burghauser R. Taste thresholds: quality specific variation with human aging. J Gerontol 1982;37:372–377.ArticlePubMed
- 36. Meyers C, Amick MA, Friedman JH. Ice cream preference in Parkinson’s disease. Med Health R I 2010;93:91–92.PubMed
- 37. Sienkiewicz-Jarosz H, Scinska A, Swiecicki L, Lipczynska-Lojkowska W, Kuran W, Ryglewicz D, et al. Sweet liking in patients with Parkinson’s disease. J Neurol Sci 2013;329:17–22.ArticlePubMed
- 38. Park K, Oeda T, Kohsaka M, Tomita S, Umemura A, Sawada H. Low body mass index and life prognosis in Parkinson’s disease. Parkinsonism Relat Disord 2018;55:81–85.ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- The missing piece of the puzzle – The key role of the dietitian in the management of Parkinson's disease
Richelle Flanagan, Carley Rusch, Fiona E. Lithander, Indu Subramanian
Parkinsonism & Related Disorders.2024; 121: 106021. CrossRef - Body mass index in patients with Parkinson’s disease: a systematic review
Yinghui Li, Yumei Liu, Chuanning Du, Jun Wang
Journal of Neurophysiology.2024; 131(2): 311. CrossRef - Apnea behavior in early- and late-stage mouse models of Parkinson's disease: Cineradiographic analysis of spontaneous breathing, acute stress, and swallowing
Lorena Roberta de Souza Mendes Kawamura, Max Sarmet, Priscila Sales de Campos, Sachiko Takehara, Yasuhiro Kumei, Jorge Luis Lopes Zeredo
Respiratory Physiology & Neurobiology.2024; 323: 104239. CrossRef - Gustatory dysfunction is related to Parkinson's disease: A systematic review and meta‐analysis
Il‐Youp Kwak, Kyung Soo Kim, Hyun Jin Min
International Forum of Allergy & Rhinology.2023; 13(10): 1949. CrossRef
Comments on this article
- Figure
- Related articles
-
- Copper Deficiency Myeloneuropathy in a Patient With Wilson’s Disease
- Fighting Against the Clock: Circadian Disruption and Parkinson’s Disease
- Absence of Alpha-Synuclein Aggregation in Patients With Parkinson’s Disease Complicated by Sigmoid Volvulus
- Caregiver Burden of Patients With Huntington’s Disease in South Korea
- A Survey of Perspectives on Telemedicine for Patients With Parkinson’s Disease
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite