Articles
- Page Path
- HOME > J Mov Disord > Volume 15(1); 2022 > Article
-
Review Article
The Supplementary Motor Complex in Parkinson’s Disease -
Shervin Rahimpour1
 , Shashank Rajkumar2
, Shashank Rajkumar2 , Mark Hallett3
, Mark Hallett3
-
Journal of Movement Disorders 2022;15(1):21-32.
DOI: https://doi.org/10.14802/jmd.21075
Published online: November 25, 2021
1Department of Neurosurgery, Clinical Neuroscience Center, University of Utah, Salt Lake City, UT, USA
2Department of Neurosurgery, Duke University Hospital, Durham, NC, USA
3Human Motor Control Section, Medical Neurology Branch, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA
- Corresponding author: Shervin Rahimpour, MD Department of Neurosurgery, Clinical Neuroscience Center, University of Utah, 175 N. Medical Drive East, Salt Lake City, UT 84132, USA / Tel: +1-8015816908 / E-mail: shervin.rahimpour@utah.edu
Copyright © 2022 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Parkinson’s disease (PD) is a neurodegenerative disorder characterized by both motor and nonmotor symptoms. Although the basal ganglia is traditionally the primary brain region implicated in this disease process, this limited view ignores the roles of the cortex and cerebellum that are networked with the basal ganglia to support motor and cognitive functions. In particular, recent research has highlighted dysfunction in the supplementary motor complex (SMC) in patients with PD. Using the PubMed and Google Scholar search engines, we identified research articles using keywords pertaining to the involvement of the SMC in action sequencing impairments, temporal processing disturbances, and gait impairment in patients with PD. A review of abstracts and full-text articles was used to identify relevant articles. In this review of 63 articles, we focus on the role of the SMC in PD, highlighting anatomical and functional data to create new perspectives in understanding clinical symptoms and, potentially, new therapeutic targets. The SMC has a nuanced role in the pathophysiology of PD, with both hypo- and hyperactivation associated with various symptoms. Further studies using more standardized patient populations and functional tasks are needed to more clearly elucidate the role of this region in the pathophysiology and treatment of PD.
- Parkinson’s disease (PD) is a chronic neurodegenerative disorder characterized by a constellation of motor and nonmotor symptoms, including resting tremor, bradykinesia, rigidity, impaired gait, and behavioral and cognitive dysfunctions. The pathological hallmarks of this constellation include a progressive loss of neurons in the substantia nigra with dopaminergic denervation of the striatum and intracytoplasmic inclusions of α-synuclein, known as Lewy bodies [1]. The traditional model of PD predicts that the loss of dopaminergic neurons in the parkinsonian state leads to increased inhibition of the indirect pathway and decreased activation of the direct pathway, causing net overactivation of the basal ganglia (BG) output [2]. This view deserves merit; to date, theoretical and empirical approaches to the treatment of PD have focused on alterations in the BG [2,3]. The BG, however, is only one part of brain networks that work to support both motor and cognitive functions, and BG-cortical-cerebellar anatomical and functional circuits are fundamental for motor tasks, sensorimotor mapping, working memory, and reasoning [4-7]. Furthermore, the traditional model of PD pathophysiology fails to comprehensively account for all symptoms of PD, such as postural imbalances and sequential motor impairments.
- Recent findings have been summarized in a theoretical framework for understanding the pathology of PD as a system-level dysfunction of the BG–cortex–cerebellum network that is not limited to the traditional paradigm of BG dysfunction alone [8]. These data have also stimulated research investigating the role of cortical areas in impairments associated with PD. For example, patients with PD commonly exhibit decreased activity in the supplementary motor complex (SMC), which improves with dopamine replacement therapy and deep brain stimulation (DBS) [9-11]. Pathologically, patients also have selective loss of pyramidal neurons of the presupplementary motor area (pre-SMA) [12]. A recent review also highlighted the pathological and compensatory effects of the cerebellum on PD [13]. In summary, studies have documented the importance of other critical areas involved in PD in addition to the BG in isolation, specifically the BG-cortex-cerebellum circuitry. Here, we focus on the role of the SMC. In this review, we discuss the functional and physical anatomy of the SMC in the context of PD, highlighting studies from both humans and monkeys. Specifically, we discuss three key symptoms of PD—action sequencing impairments, temporal processing, and gait impairment—in the context of SMC function, given its crucial role in linking cognition to action. The purpose of this review is to synthesize new perspectives for understanding PD with the ultimate goal of identifying the SMC as a potential target for innovative therapeutic approaches.
INTRODUCTION
- We searched the PubMed database and Google Scholar for relevant articles. The SMC is known to be involved in a variety of processes, including certain types of movement, executive control, and learning [14]. However, we targeted search terms to the three aforementioned symptoms (i.e., action sequence and gait impairment, and temporal processing), as they are specific symptoms of PD that have been associated with SMC dysfunction. Furthermore, searches were performed using search terms relevant to treatments for PD involving the SMC.
- In PubMed, the following search terms were used: (((Supplementary motor cortex) OR (supplementary motor area) OR (SMA) OR (SMC)) AND (Parkinson’s Disease)) AND (Treatment); (“temporal processing”) AND (“supplementary motor area”); ((“supplementary motor area” OR “supplementary motor cortex” OR “SMA”) AND (Parkinson’s Disease)) AND (sequential motor); (“Supplementary Motor Area” OR “Supplementary Motor Complex” OR “Supplementary Motor Cortex”) AND (Parkinson’s Disease). After limiting the search to the English language, 2,510 results were retrieved. Additionally, the following search terms were used in Google Scholar: “Supplementary motor area motor sequences imaging”; “Supplementary motor area” AND (“temporal processing” OR “time”) AND “supplementary motor area”; “Supplementary Motor Area” AND “gait” AND “Parkinson’s.” The results were limited to English, and the first 50 articles were evaluated for each search. No articles were excluded based on the publication date because, to our knowledge, no review has previously been published that details the role of the SMA in PD. We then reviewed the resulting 2,657 results for relevance to the present subject matter. Sixty-three articles were identified as relevant and were included in our discussion. This methodology is summarized in Figure 1.
- Anatomical connections between the BG and SMC
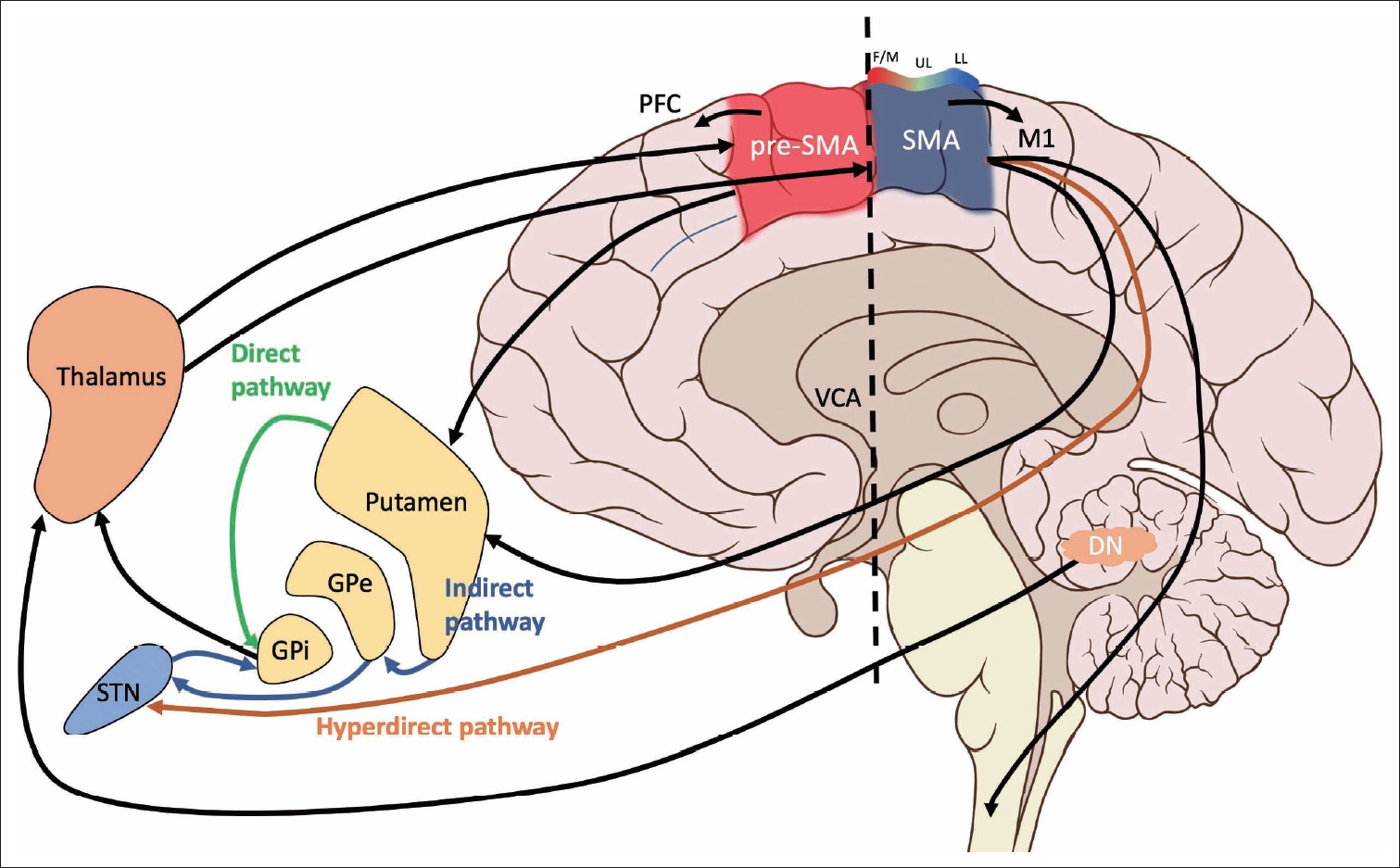
- The SMC is comprised of two functionally and cyto- and chemoarchitectonically distinct regions: the supplementary motor area (SMA) and the pre-SMA [15-20]. Early electrical stimulation studies placed these regions in the medial portion of Brodmann’s area 6 [21]. Both the SMA and pre-SMA are located in the superior frontal gyrus, rostral to the primary motor area in Brodmann’s areas 6aα and 6aβ, respectively [22]. A third region termed the supplementary eye field (SEF) lies between the SMA and pre-SMA in the upper part of the paracentral sulcus [23]. Based on more extensive human and animal studies, including clinical series, intraoperative mapping, functional and anatomical neuroimaging, and cadaveric fiber dissection, these regions have been shown to form connections with the BG, cerebellum, limbic system, thalamus, superior parietal lobe, other regions of the frontal lobe, and spinal cord [24-26]. Although they are neighboring regions, the SMA and pre-SMA exhibit differences in connectivity and functionality [15] and are not directly connected to each other [24]. The SMA sends dense direct projections to the primary motor cortex (M1) and spinal cord [27-30]. The pre-SMA, unlike the SMA, sends minimal projections to the corticospinal tract and lacks reciprocal connections with M1 [31-33]. Rather, the pre-SMA has dense connections with other areas of the prefrontal cortex [34]. Both the SMA and pre-SMA are connected with the striatum, although these projections appear to be distinct circuits [35]. Consistent with this difference in connectivity, researchers have postulated that the SMA is mainly involved in tasks relating to motor planning and execution, whereas the pre-SMA is mainly associated with cognitive processes [36]; however, the exact roles of each of these subdivisions are still under investigation. Here, we will focus on SMC connections to the BG and cerebellum in the context of PD. All areas of the SMC are targets of the BG and cerebellum [24], although the SMC receives relatively more input from the BG than the cerebellum. These projections are derived from the internal segment of the globus pallidus (GPi) via the direct and indirect pathways and the dentate nucleus via the thalamus. Regarding the cerebellum, the SMA and pre-SMA also receive inputs from spatially separate and neurochemically distinct regions of the dentate nucleus via the thalamus [24]. The pre-SMA receives input from the ventral dentate nucleus, while SMA input is more dorsal. These two subregions of the dentate nucleus designate nonmotor (ventral, pre-SMA projections) and motor (dorsal, SMA projections) domains [37]. Additionally, the hyperdirect pathway connects the SMC and the subthalamic nucleus (STN), which in turn modulates GPi activity [38]. The hyperdirect pathway is proposed to play a role in rapidly “braking” ongoing cortical-BG activity [39]. The anatomy and connectivity of the SMC are summarized in Figure 2.
- Neurophysiology and functional imaging
- Given the motor and nonmotor projections of the SMC, this cortical region may play a role in the pathophysiology of various PD symptoms. Although SMC dysfunction has been associated with symptoms such as fatigue [40], it is most commonly associated with deficits in motor preparation and execution. Here, we discuss the current state of the literature describing the role of SMC dysfunction in patients with PD as it relates to impairments of sequential movements, temporal processing, and gait. Notably, the SMC is not solely responsible for these symptoms; rather, altered interactions between the SMC and other brain areas are likely important and warrant consideration when describing circuit-level dysfunction in patients with PD. A summary of these findings is provided in Table 1.
- Action sequencing impairments
- Impairments in action sequencing are a motor deficit characterized by a slowing of sequential movements and are a common symptom of PD. In particular, when performing sequential actions, patients with PD often exhibit slowing movements and decreased amplitudes of motion, known as the sequence effect [41]. Although the physiology of the sequence effect remains unclear, this particular symptom does not completely improve with levodopa therapy, suggesting dysfunction not only in the BG but also in other brain regions, such as functionally connected cortical regions, including the SMC and the cerebellum [41]. In primate models, a series of studies has made a case not only for the participation of the SMC in motor sequences but also for different functional roles of the SMA and pre-SMA. In both areas, cells have been discovered that respond specifically to a forthcoming sequence of movements when guided by memory [42,43]. Indeed, an injection of a γ-aminobutyric acid (GABA) agonist into either the SMA or pre-SMA induced errors in memorized motor sequences but not in nonsequential movements, and these errors were corrected by providing visual cues [44]. Finally, the monkey pre-SMA contains more neurons concerned with the rank order of movements within a particular sequence, whereas the SMA proper contains more “interval-selective” neurons that link different movements within a sequence [43,45], showing that these areas are specialized to sequence movements in different ways. Furthermore, as both the pre-SMA and SMA are connected to the cerebellum via the thalamus, the cerebellum may also assist in motor sequences by contributing anticipatory activation. By anticipating future events at fast temporal scales, the cerebellum provides a feed-forward model [46,47]. The activation of pre-SMA and SMA neurons then regulates STN activation via the hyperdirect pathway with respect to the anticipated movement. Given the role of the hyperdirect pathways in movement inhibition, this signal may demarcate the end of a previous movement. As a result, anticipatory activation of the STN (bypassing the striatum with shorter conduction time) for the next movement does not occur, therefore impairing action sequencing [8].
- Similarly, in healthy humans, the SMC plays an important role in the coordination of motor sequences [48]. Increased positron emission tomography (PET) activation of the SMA is observed in healthy adults performing sequential finger movements compared to repetitive finger movements [49]. Interestingly, no further increase in SMA activity is observed as the sequences are lengthened, implicating the SMA in a separate circuit for the coordination of sequential movements, regardless of the length of these sequences. Other functional magnetic resonance imaging (fMRI) and PET-based studies have also shown greater SMA activation when participants perform sequential finger movements than repetitive, nonsequential finger movements [50,51], regardless of whether the sequential activity was performed or imagined [51]. Importantly, higher levels of complexity also activate the SMA. Toyokura et al. [52] detected an increased fMRI signal in the SMA when sequential finger movements were performed with both hands compared with either hand alone, potentially suggesting that tasks requiring increased coordination require greater SMA activity. Lesion and stimulation studies also implicate the SMA in the organization of motor sequences. For instance, Gerloff et al. [53] reported that repetitive transcranial magnetic stimulation (rTMS) over the SMA induces errors in the performance of a prelearned complex sequence of finger movements on a piano but not on simpler sequences. Similarly, Dick et al. [54] reported a case of a patient with an SMA infarct who had difficulty performing sequential tasks, similar to the sequence deficits observed in patients with PD. Together, this evidence suggests that sequential movements require a separate circuit for coordination and possibly execution compared to simple motor processes, and the SMA is an important part of this network.
- Unsurprisingly, neuroimaging studies involving patients with PD have shown that SMC dysfunction correlates with a deficit in performing sequential movements. However, in contrast to the lesion studies described above, evidence suggests that PD causes a more nuanced, circuit-level dysfunction involving the SMA. For instance, Thobois et al. [55] showed persistent activation of the SMA in patients with PD compared to healthy controls when a sequential motor task was imagined with the akinetic hand but not with the normal hand. Although the task was imagined, evidence suggests that common circuits are employed both for motor imagery and motor execution [56]. Furthermore, several studies have documented hypoactivation of the pre-SMA in patients with PD compared to healthy controls [57,58]. Meanwhile, the SMA proper is normally activated or hyperactivated in patients with PD [55,57,59], although this finding is not consistently reported [58]. This result describing SMA hyperactivation seems to contradict lesion studies in which a lack of function of the SMA correlated with sequence deficits. It also seems to contradict the result that patients with PD display SMA hypoactivation at rest compared to healthy controls [60]. Several potential explanations for this disparity have been proposed. Current studies are limited by small sample sizes, varying complexity of motor tasks, and the investigation of patients with variable disease subtypes and severities. Additionally, conflicting results regarding SMA activation may also be attributed to poor spatial resolution, as recording hyperactivation from the SMA proper and hypoactivation from the pre-SMA as one signal might skew the results. This possibility is particularly important because the pre-SMA becomes activated in movement preparation, whereas the SMA proper is activated during both movement preparation and execution [61,62], implicating these areas in distinct circuits. Finally, another explanation for the mixed pattern of SMC dysfunction is that compensatory networks in patients with PD might be responsible for the conflicting results, and these networks may become activated during different disease stages. For example, the cerebellum is hyperactivated and shows strengthened connectivity in patients with PD. Weakened striato-thalamo-cortical and striato-cerebellar connectivity in patients with PD compared to healthy control subjects may lead to compensatory increased activity in the cerebello-thalamo-cortical loop to maintain motor function at near normal levels (Figure 3) [63]. Furthermore, if baseline activity of the SMC is low in patients with late-stage PD, a greater range for increased activation during task execution may be observed, leading to a relative hyperactivation of brain regions in these patients compared to those experiencing early-stage disease.
- Temporal processing
- In addition to impairments in action sequences, patients with PD may also have difficulty with movement due to impairments in temporal processing. These impairments include symptoms such as difficulty estimating time intervals, maintaining a musical rhythm, and increased reaction time. The SMC has been shown to be important in the temporal processing necessary for these tasks [64,65], which are common symptoms of PD [66]. In healthy humans, fMRI studies provide evidence for SMA activation during tasks that require estimating time intervals [67], and this result is corroborated by the meta-analysis published by Radua et al. [68], suggesting that the SMA is activated during both the perception of time and during more difficult cognitive tasks, thus implicating a role for this structure in both cognitive effort and temporal processing. Consistent with this finding, Tanaka and Kirino [69] reported that musicians imagining a musical performance showed increased connectivity of the SMA with several brain regions, indicating that the SMA may be important for integrating necessary components of musical practice, including timing. Konoike et al. [70] showed that the SMA is involved specifically in the motor aspects of rhythm (i.e., tapping of the foot to a beat). Patients with SMA lesions also exhibit an increased reaction time and difficulty recalling prelearned rhythms, indicating a disruption of temporal processing networks [71]. Local field potential (LFP) recordings in monkeys have shown that the parkinsonian state disrupts beta band modulation, which is normally correlated with reaction time [72]. Similar conclusions have been drawn in humans with PD. Several neuroimaging studies have reported hypoactivation of the SMA in relation to temporal processing in patients with PD [70,73-75]. However, these results are not consistently reported; some studies instead report hyperactivation of the pre-SMA and SMA proper during temporal processing tasks. For example, Criaud et al. [76] found that the level of fMRI activation of the SMA, especially the pre-SMA, predicted reaction time for both patients with PD and healthy controls performing a simple reaction time task. In this context, the authors link the SMC to a proactive inhibitory network that is inappropriately hyperactivated in patients with PD [76]. One aspect of this inhibitory behavior is temporal attention. Eckert et al. [77] performed a study in which patients with PD presented increased activation of the pre-SMA compared with controls during temporally self-initiated movements, implicating a role for this structure in the temporal initiation of motor processes. Importantly, the motor task in this study was simple and not sequential, increasing the likelihood that the increased activity was actually due to temporal self-initiation rather than coordinating a sequential task. As one might expect, differences in activation of the SMA proper and pre-SMA have been observed in regard to temporal processing. Schwartze et al. [78] summarized that sensory, nonsequential, and suprasecond temporal processing generally activate the pre-SMA, whereas the SMA proper is generally more highly activated in sensorimotor, sequential, and subsecond temporal processing. Thus, the anatomically different sections of the SMC may be part of functionally distinct circuits that contribute to different aspects of time perception. This result is particularly important when we consider the potential role of compensatory networks activated in patients with PD due to dysfunction of the BG-thalamocortical network [79].
- Importantly, some evidence suggests a different source for temporal processing. Conte et al. [80] found that continuous theta burst stimulation (cTBS) over the primary somatosensory cortex but not the pre-SMA affected temporal processing in healthy volunteers, and Koch et al. [81] reported improved performance on a time reproduction task in patients with PD when rTMS was applied over the dorsolateral prefrontal cortex but not over the SMA. Nevertheless, the evidence generally implicates the SMA in the timing of motor processes.
- Importantly, deficits in temporal processing impair not only the initiation and timing of motor processes in patients with PD but also speech and language processing. Speech and language strongly rely on processing accurate timing intervals. A recent review of nonmotor functions of the BG suggests that the pre-SMA-BG circuit is involved in the temporal processing of speech and synchronization of temporal aspects of speech with syntax in language processing [82].
- Together, strong evidence indicates that the SMC is at least part of a circuit that helps the brain perceive time intervals, and the pre-SMA and SMA proper have distinct roles in this function. Furthermore, in patients with PD, dysfunction of the SMC impairs temporal processing, leading to difficulty in initiating movements and altered perception of discrete time intervals. More work is needed to properly elucidate the roles of primary SMC dysfunction and SMA hyperactivation as a compensatory mechanism in these patients.
- Gait impairment
- Gait impairment is another common disabling motor symptom of PD, and the SMC has been implicated in this pathology in several ways. First, functional studies suggest that hypoactivation of the SMA correlates with the slowing of volitional finger movements [83,84], suggesting that SMA dysfunction might cause slowing of gait. Additionally, gait involves a series of sequential lower extremity movements, and as previously discussed, SMC dysfunction is implicated in problems in executing sequential motor plans. Disruption of the SMA has also been shown to alter the generation of anticipatory postural adjustments prior to gait initiaton [85]. Finally, the SMA has also been implicated in freezing of gait (FOG), a common type of gait impairment in patients with PD. For instance, decreased activity in the SMA was observed in patients with PD presenting FOG compared to those without FOG during motor imagery in a gait task [86]. Although this result did not reach significance, several interventional studies targeting the SMC with rTMS have shown a clinical improvement in FOG [87,88]. Importantly, however, the current literature only implicates the dysfunction—not specifically the hypoactivation—of the SMC in gait impairment. Freezing of upper limb movement was associated with increased SMC activity, and transcranial direct current stimulation of the SMC in one study did not improve FOG [89]. Based on these results, our current understanding of the exact role of the SMC in gait is still limited. This finding is corroborated by the review by Snijders et al. [90], which highlights the importance of the dynamic interplay of different neural networks and the hyper and hypoactivation of the SMC in the disease state. Furthermore, heterogeneity in clinical subtypes of PD may lead to different activation patterns.
- SMC as a target for PD treatment
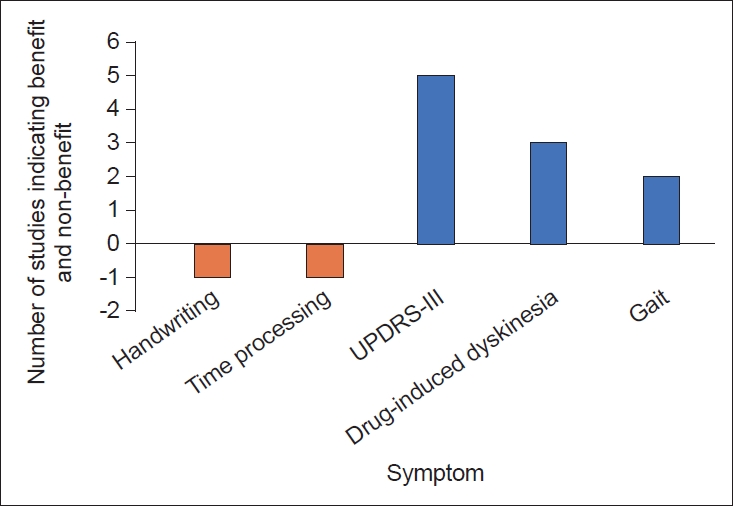
- Since the SMC has been implicated in many symptoms of PD, studies have targeted the SMA for the treatment of PD. Medical therapies for PD, including dopamine agonists, have been shown to alter the activity of the SMC [75,91]. Furthermore, studies have examined fMRI-guided neurofeedback as a potential treatment strategy for patients with PD and have found that this strategy increases SMA activity and improves symptoms [92-94]. However, these interventions are nonspecific to modulation of the SMC and are known to act on other regions, such as the BG. We therefore turned our attention to neuromodulation specifically targeting the SMC, which has also been shown to effectively alleviate symptoms of PD. Benefits of direct cortical stimulation in a patient with PD was first observed in 1979 by Woolsey et al. [95] Subthreshold stimulation of the precentral gyrus resulted in a transient disappearance of tremor and rigidity. More recently, the evidence has shown efficacy in alleviating tremors in patients receiving M1 stimulation for the treatment of chronic pain [96]. Noninvasive methods with transcranial magnetic stimulation (TMS) specifically targeting the SMC have also been used, providing more definitive evidence of an antiparkinsonian effect (Table 2). However, cortical stimulation of areas including the SMA remains poorly understood [97]. Current studies involve a limited number of patients and vary in the frequency of stimulation used, the duration of treatment and number of pulses, and in the measurement of clinical improvement after stimulation. This variability may explain why evidence regarding the efficacy rTMS of the SMC has yielded mixed results. Notably, neuromodulation of other cortical areas may also be beneficial; however, the amelioration of symptoms in some of these studies (e.g., three studies investigating 1 Hz stimulation found a reduction in drug-induced dyskinesias) warrants further investigation into the role of neuromodulation of the SMC as a potential therapy. A summary of the results of cortical stimulation studies of the SMC is shown in Figure 4. Based on the functional role of the SMC and the studies discussed in this review, one might imagine several other neuromodulatory therapies to achieve clinical benefit in patients with PD. For instance, cortical epidural or subdural implants with separate pre-SMA and SMA contacts for differential stimulation could be considered, enabling finer control over distinct circuits that may serve different functions. For instance, action sequencing impairments may be caused by dysfunction in the inhibitory hyperdirect pathway [8]; neuromodulation of the SMA may then represent a treatment for this symptom. Regarding DBS, studies have utilized fMRI or PET to report changes in the cortical activity of patients undergoing STN-DBS [74,98-100]. Following STN stimulation, blood oxygenation level dependent (BOLD) activation is observed in the ipsilateral SMC. While SMC activity may serve as a surrogate marker for symptoms of PD related to impairments in action sequences, temporal processing and gait impairments, the temporal resolution of fMRI is limited. Measuring SMC activity with subdural electrocorticography provides the spatial and temporal resolution necessary to observe changes in cortical activity relative to symptom onset. This input can then drive DBS through a feedback mechanism. Recently, beta-gamma phase-amplitude coupling (PAC) has been identified as a promising electrophysiological biomarker of the parkinsonian motor state that has been detected in the cortical node of the BG-thalamo-cortical network [101]. The SMC and premotor areas exhibit this abnormal coupling between β and broadband-γ and can therefore serve as a biomarker of PD. Measuring abnormal PAC in the SMC is thus useful as a control signal for a “closedloop” DBS device [102]. In this case, one could use the PAC signal to tune DBS parameters and suppress this abnormal coupling phenomenon. Excitingly, these theories have begun to move toward clinical application, as a successful feasibility study for closed loop DBS in 13 patients with PD has recently been published [103].
METHODS
- A restricted view of PD as primarily a disease of BG dysfunction ignores the effect of some cortical circuits, such as those involving the SMC, on PD symptoms. The SMC plays a role in motor sequencing, temporal processing, and gait, and the evidence presented in this review highlights that dysfunction of this cortical area is associated with impairments in each of these processes in patients with PD. Nonetheless, current evidence does not provide a clear pattern of SMC dysfunction in patients PD. Factors contributing to the differing results include differential hyper and hypoactivation of parts of the SMC in patients with PD, compensatory networks that are activated at different points during disease progression, and disparate patient populations and experimental tasks. The results of preliminary stimulation studies targeting the SMC show potential in alleviating the debilitating symptoms of PD (i.e., gait impairments); thus, future research designed to better understand SMC activity as a function of the PD phenotype and disease progression may facilitate the development of novel treatment strategies and targets.
Conclusions
-
Conflicts of Interest
The authors have no financial conflicts of interest.
-
Funding Statement
None.
-
Author Contributions
Conceptualization: Shervin Rahimpour, Mark Hallett. Data curation: all authors. Formal analysis: all authors. Visualization: Shervin Rahimpour, Mark Hallett. Writing—original draft: all authors. Writing—review & editing: all authors.
Notes


The effects of SMC lesions and SMC stimulation on action sequencing impairment, temporal processing, and gait impairment. Overall, SMC lesions worsen performance on these tasks, while stimulation may worsen action sequences and temporal processing but improve gait impairment. Functional imaging reveals differential activation of the SMA and pre-SMA when these tasks are performed. SMC, supplementary motor complex; SMA, supplementary motor area; pre-SMA, presupplementary motor area; FOG, freezing of gait.
| Study | Patients (n) | Stimulation parameters (frequency, intensity, number of pulses) | Results |
|---|---|---|---|
| Boylan et al. [104] | 10 | 10-Hz, 110% MT, 2,000 | Worsening of reaction times and handwriting |
| Koch et al. [81] | 10 | 5-Hz, 100% MT, 250 | No significant effect on time perception |
| Koch et al. [105] | 8 | 1-Hz, 90% RMT, 900 | 1-Hz markedly reduced drug-induced dyskinesias, 5-Hz rTMS induced a slight but not significant increase |
| 5-Hz, 110% RMT, 900 | |||
| Brusa et al. [106] | 10 | 1-Hz, 90% RMT, 900 | Reduction in levodopa-induced dyskinesia |
| Hamada et al. [107] | 98 (55 active, 43 sham) | 5-Hz, 110% AMT, 1,000 (1 day)/week × 8 weeks | Improvements in total and motor UPDRS |
| Hamada et al. [88] | 98 (55 active, 43 sham) | 5-Hz, 110% AMT, 1,000 (1 day)/week × 8 weeks | A subgroup analysis of UPDRS revealed improved bradykinesia in patients with PD |
| Shirota et al. [108]* | 106 (36 active 1-Hz, 34 active 10-Hz, and 36 sham) | 1-Hz, 110% AMT/110% RMT, 1,000 (1 day)/week × 8 weeks | Improvement of motor UPDRS in the 1-Hz group. Sham stimulation and 10-Hz rTMS transiently improved motor symptoms, but effects disappeared during the observation period. |
| 10-Hz, 110% AMT/110% RMT, 1,000 (1 day)/week × 8 weeks | |||
| Kim et al. [87] | 12 | 25-Hz, 100% RMT, 100/day × 2 days | Improved gait and fewer freezing episodes during SMA stimulation compared to motor cortex stimulation |
| Sayın et al. [109] | 17 (9 treatment, 8 sham) | 1-Hz, 90% RMT, 1,800/day × 10 days | Decreased levodopa-induced dyskinesia for 24 hours with no change in motor function for SMA stimulation compared with sham stimulation |
| Jacobs et al. [85] | 16 (8 with PD, 8 without PD) | 1-Hz, 80% RMT, 1,800/day × 1 day | Decreased duration but not amplitude of APA in both groups. The symptom severity of patients with PD was positively correlated with the extent to which their APA duration was changed |
| Yokoe et al. [110] | 19 | 10-Hz, 100% RMT, 1,000 (1 day)/week × 12 weeks | Significant change in UPDRS-III after SMA and M1 stimulation (better with M1, although not significant). Significant amelioration of upper limb scores after stimulation over M1 and SMA and of akinesia after stimulation of M1. |
| Ma et al. [111] | 28 | 10-Hz, 90% RMT, 1,000 (1 day)/week × 2 weeks | No change in sequence effect. Improvements in FOG, UPDRS-III, ambulation time, cadence, step count, and velocity with real stimulation. |
| Lee et al. [112] | 10 | 10-Hz, 90% RMT, 1,000 | Lack of improvement with SMC stimulation, but improvement was observed after motor cortex and DLPFC stimulation. |
All studies included some form of a sham control.
* randomized, double-blind, sham-controlled, multicenter trial.
PD, Parkinson’s disease; rTMS, repetitive transcranial magnetic stimulation; SMA, supplementary motor area; APA, anticipatory postural adjustment; M1, primary motor cortex; FOG, freezing of gait; SMC, supplementary motor complex; MT, motor threshold; RMT, resting motor threshold; AMT, active motor threshold; UPDRS, Unified Parkinson’s Disease Rating Scale; DLPFC, dorsolateral prefrontal cortex.
- 1. Dickson DW. Neuropathology of Parkinson disease. Parkinsonism Relat Disord 2018;46 Suppl 1:S30–S33.ArticlePubMed
- 2. Obeso JA, Rodríguez-Oroz MC, Rodríguez M, Lanciego JL, Artieda J, Gonzalo N, et al. Pathophysiology of the basal ganglia in Parkinson’s disease. Trends Neurosci 2000;23:S8–S19.ArticlePubMed
- 3. Wiecki TV, Frank MJ. Neurocomputational models of motor and cognitive deficits in Parkinson’s disease. Prog Brain Res 2010;183:275–297.ArticlePubMed
- 4. Caligiore D, Pezzulo G, Baldassarre G, Bostan AC, Strick PL, Doya K, et al. Consensus paper: towards a systems-level view of cerebellar function: the interplay between cerebellum, basal ganglia, and cortex. Cerebellum 2017;16:203–229.ArticlePubMed
- 5. Jahanshahi M, Obeso I, Rothwell JC, Obeso JA. A fronto-striato-subthalamic-pallidal network for goal-directed and habitual inhibition. Nat Rev Neurosci 2015;16:719–732.ArticlePubMed
- 6. McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci 2008;11:103–107.ArticlePubMed
- 7. Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci 2006;7:464–476.ArticlePubMed
- 8. Caligiore D, Helmich RC, Hallett M, Moustafa AA, Timmermann L, Toni I, et al. Parkinson’s disease as a system-level disorder. NPJ Parkinsons Dis 2016;2:16025.ArticlePubMedPMC
- 9. Grafton ST. Contributions of functional imaging to understanding parkinsonian symptoms. Curr Opin Neurobiol 2004;14:715–719.ArticlePubMed
- 10. Haslinger B, Erhard P, Kämpfe N, Boecker H, Rummeny E, Schwaiger M, et al. Event-related functional magnetic resonance imaging in Parkinson’s disease before and after levodopa. Brain 2001;124:558–570.ArticlePubMed
- 11. Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RS, Brooks DJ. Impaired mesial frontal and putamen activation in Parkinson’s disease: a positron emission tomography study. Ann Neurol 1992;32:151–161.ArticlePubMed
- 12. MacDonald V, Halliday GM. Selective loss of pyramidal neurons in the pre-supplementary motor cortex in Parkinson’s disease. Mov Disord 2002;17:1166–1173.ArticlePubMed
- 13. Wu T, Hallett M. The cerebellum in Parkinson’s disease. Brain 2013;136:696–709.ArticlePubMedPMC
- 14. Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci 2008;9:856–869.ArticlePubMed
- 15. Kim JH, Lee JM, Jo HJ, Kim SH, Lee JH, Kim ST, et al. Defining functional SMA and pre-SMA subregions in human MFC using resting state fMRI: functional connectivity-based parcellation method. Neuroimage 2010;49:2375–2386.ArticlePubMed
- 16. Luppino G, Matelli M, Rizzolatti G. Cortico-cortical connections of two electrophysiologically identified arm representations in the mesial agranular frontal cortex. Exp Brain Res 1990;82:214–218.ArticlePubMed
- 17. Luppino G, Matelli M, Camarda RM, Gallese V, Rizzolatti G. Multiple representations of body movements in mesial area 6 and the adjacent cingulate cortex: an intracortical microstimulation study in the macaque monkey. J Comp Neurol 1991;311:463–482.ArticlePubMed
- 18. Matsuzaka Y, Aizawa H, Tanji J. A motor area rostral to the supplementary motor area (presupplementary motor area) in the monkey: neuronal activity during a learned motor task. J Neurophysiol 1992;68:653–662.ArticlePubMed
- 19. Tanji J. The supplementary motor area in the cerebral cortex. Neurosci Res 1994;19:251–268.ArticlePubMed
- 20. Tanji J. New concepts of the supplementary motor area. Curr Opin Neurobiol 1996;6:782–787.ArticlePubMed
- 21. Penfield W, Welch K. The supplementary motor area of the cerebral cortex; a clinical and experimental study. AMA Arch Neurol Psychiatry 1951;66:289–317.PubMed
- 22. Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex 1996;6:342–353.ArticlePubMed
- 23. Grosbras MH, Lobel E, Van de Moortele PF, LeBihan D, Berthoz A. An anatomical landmark for the supplementary eye fields in human revealed with functional magnetic resonance imaging. Cereb Cortex 1999;9:705–711.ArticlePubMed
- 24. Akkal D, Dum RP, Strick PL. Supplementary motor area and presupplementary motor area: targets of basal ganglia and cerebellar output. J Neurosci 2007;27:10659–10673.ArticlePubMedPMC
- 25. Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, WheelerKingshott CA, Boulby PA, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 2003;6:750–757.ArticlePubMed
- 26. Bozkurt B, Yagmurlu K, Middlebrooks EH, Karadag A, Ovalioglu TC, Jagadeesan B, et al. Microsurgical and tractographic anatomy of the supplementary motor area complex in humans. World Neurosurg 2016;95:99–107.ArticlePubMed
- 27. Dum RP, Strick PL. Spinal cord terminations of the medial wall motor areas in macaque monkeys. J Neurosci 1996;16:6513–6525.ArticlePubMedPMC
- 28. Dum RP, Strick PL. Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere. J Neurosci 2005;25:1375–1386.ArticlePubMedPMC
- 29. Maier MA, Armand J, Kirkwood PA, Yang HW, Davis JN, Lemon RN. Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: an anatomical and electrophysiological study. Cereb Cortex 2002;12:281–296.ArticlePubMed
- 30. Muakkassa KF, Strick PL. Frontal lobe inputs to primate motor cortex: evidence for four somatotopically organized ‘premotor’ areas. Brain Res 1979;177:176–182.ArticlePubMed
- 31. Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci 1991;11:667–689.ArticlePubMedPMC
- 32. Luppino G, Matelli M, Camarda R, Rizzolatti G. Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J Comp Neurol 1993;338:114–140.ArticlePubMed
- 33. Luppino G, Matelli M, Camarda R, Rizzolatti G. Corticospinal projections from mesial frontal and cingulate areas in the monkey. Neuroreport 1994;5:2545–2548.ArticlePubMed
- 34. Wang Y, Isoda M, Matsuzaka Y, Shima K, Tanji J. Prefrontal cortical cells projecting to the supplementary eye field and presupplementary motor area in the monkey. Neurosci Res 2005;53:1–7.ArticlePubMed
- 35. Lehéricy S, Ducros M, Krainik A, Francois C, Van de Moortele PF, Ugurbil K, et al. 3-D diffusion tensor axonal tracking shows distinct SMA and pre-SMA projections to the human striatum. Cereb Cortex 2004;14:1302–1309.ArticlePubMed
- 36. Zhang S, Ide JS, Li CS. Resting-state functional connectivity of the medial superior frontal cortex. Cereb Cortex 2012;22:99–111.ArticlePubMed
- 37. Küper M, Dimitrova A, Thürling M, Maderwald S, Roths J, Elles HG, et al. Evidence for a motor and a non-motor domain in the human dentate nucleus--an fMRI study. Neuroimage 2011;54:2612–2622.ArticlePubMed
- 38. Nambu A, Tokuno H, Takada M. Functional significance of the corticosubthalamo-pallidal ‘hyperdirect’ pathway. Neurosci Res 2002;43:111–117.ArticlePubMed
- 39. Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science 2007;318:1309–1312.ArticlePubMed
- 40. Siciliano M, De Micco R, Giordano A, Di Nardo F, Russo A, Caiazzo G, et al. Supplementary motor area functional connectivity in “drug-naïve” Parkinson’s disease patients with fatigue. J Neural Transm (Vienna) 2020;127:1133–1142.ArticlePubMed
- 41. Bologna M, Paparella G, Fasano A, Hallett M, Berardelli A. Evolving concepts on bradykinesia. Brain 2020;143:727–750.ArticlePubMed
- 42. Tanji J, Shima K. Role for supplementary motor area cells in planning several movements ahead. Nature 1994;371:413–416.ArticlePubMed
- 43. Shima K, Tanji J. Neuronal activity in the supplementary and presupplementary motor areas for temporal organization of multiple movements. J Neurophysiol 2000;84:2148–2160.ArticlePubMed
- 44. Shima K, Tanji J. Both supplementary and presupplementary motor areas are crucial for the temporal organization of multiple movements. J Neurophysiol 1998;80:3247–3260.ArticlePubMed
- 45. Isoda M, Tanji J. Participation of the primate presupplementary motor area in sequencing multiple saccades. J Neurophysiol 2004;92:653–659.ArticlePubMed
- 46. Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci 2009;32:413–434.ArticlePubMed
- 47. Caligiore D, Pezzulo G, Miall RC, Baldassarre G. The contribution of brain sub-cortical loops in the expression and acquisition of action understanding abilities. Neurosci Biobehav Rev 2013;37:2504–2515.ArticlePubMedPMC
- 48. Roland PE, Larsen B, Lassen NA, Skinhøj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol 1980;43:118–136.ArticlePubMed
- 49. Catalan MJ, Honda M, Weeks RA, Cohen LG, Hallett M. The functional neuroanatomy of simple and complex sequential finger movements: a PET study. Brain 1998;121:253–264.ArticlePubMed
- 50. Shibasaki H, Sadato N, Lyshkow H, Yonekura Y, Honda M, Nagamine T, et al. Both primary motor cortex and supplementary motor area play an important role in complex finger movement. Brain 1993;116:1387–1398.ArticlePubMed
- 51. Rao SM, Binder JR, Bandettini PA, Hammeke TA, Yetkin FZ, Jesmanowicz A, et al. Functional magnetic resonance imaging of complex human movements. Neurology 1993;43:2311–2318.ArticlePubMed
- 52. Toyokura M, Muro I, Komiya T, Obara M. Activation of pre-supplementary motor area (SMA) and SMA proper during unimanual and bimanual complex sequences: an analysis using functional magnetic resonance imaging. J Neuroimaging 2002;12:172–178.ArticlePubMed
- 53. Gerloff C, Corwell B, Chen R, Hallett M, Cohen LG. Stimulation over the human supplementary motor area interferes with the organization of future elements in complex motor sequences. Brain 1997;120:1587–1602.ArticlePubMed
- 54. Dick JP, Benecke R, Rothwell JC, Day BL, Marsden CD. Simple and complex movements in a patient with infarction of the right supplementary motor area. Mov Disord 1986;1:255–266.ArticlePubMed
- 55. Thobois S, Dominey PF, Decety J, Pollak PP, Gregoire MC, Le Bars PD, et al. Motor imagery in normal subjects and in asymmetrical Parkinson’s disease: a PET study. Neurology 2000;55:996–1002.ArticlePubMed
- 56. Dominey P, Decety J, Broussolle E, Chazot G, Jeannerod M. Motor imagery of a lateralized sequential task is asymmetrically slowed in hemiParkinson’s patients. Neuropsychologia 1995;33:727–741.ArticlePubMed
- 57. Sabatini U, Boulanouar K, Fabre N, Martin F, Carel C, Colonnese C, et al. Cortical motor reorganization in akinetic patients with Parkinson’s disease: a functional MRI study. Brain 2000;123:394–403.ArticlePubMed
- 58. Mallol R, Barrós-Loscertales A, López M, Belloch V, Parcet MA, Avila C. Compensatory cortical mechanisms in Parkinson’s disease evidenced with fMRI during the performance of pre-learned sequential movements. Brain Res 2007;1147:265–271.ArticlePubMed
- 59. Caproni S, Muti M, Principi M, Ottaviano P, Frondizi D, Capocchi G, et al. Complexity of motor sequences and cortical reorganization in Parkinson’s disease: a functional MRI study. PLoS One 2013;8:e66834. ArticlePubMedPMC
- 60. Wu T, Wang L, Chen Y, Zhao C, Li K, Chan P. Changes of functional connectivity of the motor network in the resting state in Parkinson’s disease. Neurosci Lett 2009;460:6–10.ArticlePubMed
- 61. Lee KM, Chang KH, Roh JK. Subregions within the supplementary motor area activated at different stages of movement preparation and execution. Neuroimage 1999;9:117–123.ArticlePubMed
- 62. Halsband U, Matsuzaka Y, Tanji J. Neuronal activity in the primate supplementary, pre-supplementary and premotor cortex during externally and internally instructed sequential movements. Neurosci Res 1994;20:149–155.ArticlePubMed
- 63. Wu T, Chan P, Hallett M. Effective connectivity of neural networks in automatic movements in Parkinson’s disease. Neuroimage 2010;49:2581–2587.ArticlePubMed
- 64. Macar F, Lejeune H, Bonnet M, Ferrara A, Pouthas V, Vidal F, et al. Activation of the supplementary motor area and of attentional networks during temporal processing. Exp Brain Res 2002;142:475–485.ArticlePubMed
- 65. Ferrandez AM, Hugueville L, Lehéricy S, Poline JB, Marsault C, Pouthas V. Basal ganglia and supplementary motor area subtend duration perception: an fMRI study. Neuroimage 2003;19:1532–1544.ArticlePubMed
- 66. Schwartze M, Kotz SA. Regional interplay for temporal processing in Parkinson’s disease: possibilities and challenges. Front Neurol 2016;6:270.ArticlePubMedPMC
- 67. Macar F, Anton JL, Bonnet M, Vidal F. Timing functions of the supplementary motor area: an event-related fMRI study. Brain Res Cogn Brain Res 2004;21:206–215.ArticlePubMed
- 68. Radua J, Del Pozo NO, Gómez J, Guillen-Grima F, Ortuño F. Meta-analysis of functional neuroimaging studies indicates that an increase of cognitive difficulty during executive tasks engages brain regions associated with time perception. Neuropsychologia 2014;58:14–22.ArticlePubMed
- 69. Tanaka S, Kirino E. Dynamic reconfiguration of the supplementary motor area network during imagined music performance. Front Hum Neurosci 2017;11:606.ArticlePubMedPMC
- 70. Konoike N, Kotozaki Y, Jeong H, Miyazaki A, Sakaki K, Shinada T, et al. Temporal and motor representation of rhythm in fronto-parietal cortical areas: an fMRI study. PLoS One 2015;10:e0130120. ArticlePubMedPMC
- 71. Halsband U, Ito N, Tanji J, Freund HJ. The role of premotor cortex and the supplementary motor area in the temporal control of movement in man. Brain 1993;116:243–266.ArticlePubMed
- 72. Hendrix CM, Campbell BA, Tittle BJ, Johnson LA, Baker KB, Johnson MD, et al. Predictive encoding of motor behavior in the supplementary motor area is disrupted in parkinsonism. J Neurophysiol 2018;120:1247–1255.ArticlePubMedPMC
- 73. Buhmann C, Glauche V, Stürenburg HJ, Oechsner M, Weiller C, Büchel C. Pharmacologically modulated fMRI--cortical responsiveness to levodopa in drug-naive hemiparkinsonian patients. Brain 2003;126:451–461.ArticlePubMed
- 74. Ceballos-Baumann AO, Boecker H, Bartenstein P, von Falkenhayn I, Riescher H, Conrad B, et al. A positron emission tomographic study of subthalamic nucleus stimulation in Parkinson disease: enhanced movement-related activity of motor-association cortex and decreased motor cortex resting activity. Arch Neurol 1999;56:997–1003.ArticlePubMed
- 75. Jenkins IH, Fernandez W, Playford ED, Lees AJ, Frackowiak RS, Passingham RE, et al. Impaired activation of the supplementary motor area in Parkinson’s disease is reversed when akinesia is treated with apomorphine. Ann Neurol 1992;32:749–757.ArticlePubMed
- 76. Criaud M, Poisson A, Thobois S, Metereau E, Redouté J, Ibarrola D, et al. Slowness in movement initiation is associated with proactive inhibitory network dysfunction in Parkinson’s disease. J Parkinsons Dis 2016;6:433–440.ArticlePubMed
- 77. Eckert T, Peschel T, Heinze HJ, Rotte M. Increased pre-SMA activation in early PD patients during simple self-initiated hand movements. J Neurol 2006;253:199–207.ArticlePubMed
- 78. Schwartze M, Rothermich K, Kotz SA. Functional dissociation of preSMA and SMA-proper in temporal processing. Neuroimage 2012;60:290–298.ArticlePubMed
- 79. Lesiuk T, Bugos JA, Murakami B. A rationale for music training to enhance executive functions in Parkinson’s disease: an overview of the problem. Healthcare (Basel) 2018;6:35.ArticlePubMedPMC
- 80. Conte A, Rocchi L, Nardella A, Dispenza S, Scontrini A, Khan N, et al. Theta-burst stimulation-induced plasticity over primary somatosensory cortex changes somatosensory temporal discrimination in healthy humans. PLoS One 2012;7:e32979. ArticlePubMedPMC
- 81. Koch G, Oliveri M, Brusa L, Stanzione P, Torriero S, Caltagirone C. High-frequency rTMS improves time perception in Parkinson disease. Neurology 2004;63:2405–2406.ArticlePubMed
- 82. Kotz SA, Schwartze M, Schmidt-Kassow M. Non-motor basal ganglia functions: a review and proposal for a model of sensory predictability in auditory language perception. Cortex 2009;45:982–990.ArticlePubMed
- 83. Martin JA, Zimmermann N, Scheef L, Jankowski J, Paus S, Schild HH, et al. Disentangling motor planning and motor execution in unmedicated de novo Parkinson’s disease patients: an fMRI study. Neuroimage Clin 2019;22:101784.ArticlePubMedPMC
- 84. Brugger F, Wegener R, Walch J, Galovic M, Hägele-Link S, Bohlhalter S, et al. Altered activation and connectivity of the supplementary motor cortex at motor initiation in Parkinson’s disease patients with freezing. Clin Neurophysiol 2020;131:2171–2180.ArticlePubMed
- 85. Jacobs JV, Lou JS, Kraakevik JA, Horak FB. The supplementary motor area contributes to the timing of the anticipatory postural adjustment during step initiation in participants with and without Parkinson’s disease. Neuroscience 2009;164:877–885.ArticlePubMed
- 86. Peterson DS, Pickett KA, Duncan R, Perlmutter J, Earhart GM. Gait-related brain activity in people with Parkinson disease with freezing of gait. PLoS One 2014;9:e90634. ArticlePubMedPMC
- 87. Kim SJ, Paeng SH, Kang SY. Stimulation in supplementary motor area versus motor cortex for freezing of gait in Parkinson’s disease. J Clin Neurol 2018;14:320–326.ArticlePubMedPMC
- 88. Hamada M, Ugawa Y, Tsuji S; Effectiveness of rTMS on Parkinson’s Disease Study Group. High-frequency rTMS over the supplementary motor area improves bradykinesia in Parkinson’s disease: subanalysis of double-blind sham-controlled study. J Neurol Sci 2009;287:143–146.ArticlePubMed
- 89. Lu C, Amundsen Huffmaster SL, Tuite PJ, MacKinnon CD. The effects of anodal tDCS over the supplementary motor area on gait initiation in Parkinson’s disease with freezing of gait: a pilot study. J Neurol 2018;265:2023–2032.ArticlePubMedPMC
- 90. Snijders AH, Takakusaki K, Debu B, Lozano AM, Krishna V, Fasano A, et al. Physiology of freezing of gait. Ann Neurol 2016;80:644–659.ArticlePubMed
- 91. Rascol O, Sabatini U, Chollet F, Fabre N, Senard JM, Montastruc JL, et al. Normal activation of the supplementary motor area in patients with Parkinson’s disease undergoing long-term treatment with levodopa. J Neurol Neurosurg Psychiatry 1994;57:567–571.ArticlePubMedPMC
- 92. Subramanian L, Hindle JV, Johnston S, Roberts MV, Husain M, Goebel R, et al. Real-time functional magnetic resonance imaging neurofeedback for treatment of Parkinson’s disease. J Neurosci 2011;31:16309–16317.ArticlePubMedPMC
- 93. Mehler DMA, Williams AN, Krause F, Lührs M, Wise RG, Turner DL, et al. The BOLD response in primary motor cortex and supplementary motor area during kinesthetic motor imagery based graded fMRI neurofeedback. Neuroimage 2019;184:36–44.ArticlePubMed
- 94. Subramanian L, Morris MB, Brosnan M, Turner DL, Morris HR, Linden DE. Functional magnetic resonance imaging neurofeedback-guided motor imagery training and motor training for Parkinson’s disease: randomized trial. Front Behav Neurosci 2016;10:111.ArticlePubMedPMC
- 95. Woolsey CN, Erickson TC, Gilson WE. Localization in somatic sensory and motor areas of human cerebral cortex as determined by direct recording of evoked potentials and electrical stimulation. J Neurosurg 1979;51:476–506.ArticlePubMed
- 96. Meglio M, Cioni B. Motor cortex stimulation for Parkinson’s disease. In: Lozano AM, Gildenberg PL, Tasker RR, editors. Textbook of Stereotactic and Functional Neurosurgery. Berlin: Springer, Berlin, Heidelberg; 2009:1679–1690.
- 97. Lefaucheur JP. Treatment of Parkinson’s disease by cortical stimulation. Expert Rev Neurother 2009;9:1755–1771.ArticlePubMed
- 98. Phillips MD, Baker KB, Lowe MJ, Tkach JA, Cooper SE, Kopell BH, et al. Parkinson disease: pattern of functional MR imaging activation during deep brain stimulation of subthalamic nucleus--initial experience. Radiology 2006;239:209–216.ArticlePubMed
- 99. Arantes PR, Cardoso EF, Barreiros MA, Teixeira MJ, Gonçalves MR, Barbosa ER, et al. Performing functional magnetic resonance imaging in patients with Parkinson’s disease treated with deep brain stimulation. Mov Disord 2006;21:1154–1162.ArticlePubMed
- 100. Knight EJ, Testini P, Min HK, Gibson WS, Gorny KR, Favazza CP, et al. Motor and nonmotor circuitry activation induced by subthalamic nucleus deep brain stimulation in patients with Parkinson disease: intraoperative functional magnetic resonance imaging for deep brain stimulation. Mayo Clin Proc 2015;90:773–785.ArticlePubMed
- 101. Hwang BY, Salimpour Y, Tsehay YK, Anderson WS, Mills KA. Perspective: phase amplitude coupling-based phase-dependent neuromodulation in Parkinson’s disease. Front Neurosci 2020;14:558967.ArticlePubMedPMC
- 102. de Hemptinne C, Swann NC, Ostrem JL, Ryapolova-Webb ES, San Luciano M, Galifianakis NB, et al. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson’s disease. Nat Neurosci 2015;18:779–786.ArticlePubMedPMC
- 103. Velisar A, Syrkin-Nikolau J, Blumenfeld Z, Trager MH, Afzal MF, Prabhakar V, et al. Dual threshold neural closed loop deep brain stimulation in Parkinson disease patients. Brain Stimul 2019;12:868–876.ArticlePubMed
- 104. Boylan LS, Pullman SL, Lisanby SH, Spicknall KE, Sackeim HA. Repetitive transcranial magnetic stimulation to SMA worsens complex movements in Parkinson’s disease. Clin Neurophysiol 2001;112:259–264.ArticlePubMed
- 105. Koch G, Brusa L, Caltagirone C, Peppe A, Oliveri M, Stanzione P, et al. rTMS of supplementary motor area modulates therapy-induced dyskinesias in Parkinson disease. Neurology 2005;65:623–625.ArticlePubMed
- 106. Brusa L, Versace V, Koch G, Iani C, Stanzione P, Bernardi G, et al. Low frequency rTMS of the SMA transiently ameliorates peak-dose LID in Parkinson’s disease. Clin Neurophysiol 2006;117:1917–1921.ArticlePubMed
- 107. Hamada M, Ugawa Y, Tsuji S; Effectiveness of rTMS on Parkinson’s Disease Study Group. High-frequency rTMS over the supplementary motor area for treatment of Parkinson’s disease. Mov Disord 2008;23:1524–1531.ArticlePubMed
- 108. Shirota Y, Ohtsu H, Hamada M, Enomoto H, Ugawa Y; Research Committee on rTMS Treatment of Parkinson’s Disease. Supplementary motor area stimulation for Parkinson disease: a randomized controlled study. Neurology 2013;80:1400–1405.ArticlePubMed
- 109. Sayın S, Cakmur R, Yener GG, Yaka E, Uğurel B, Uzunel F. Low-frequency repetitive transcranial magnetic stimulation for dyskinesia and motor performance in Parkinson’s disease. J Clin Neurosci 2014;21:1373–1376.ArticlePubMed
- 110. Yokoe M, Mano T, Maruo T, Hosomi K, Shimokawa T, Kishima H, et al. The optimal stimulation site for high-frequency repetitive transcranial magnetic stimulation in Parkinson’s disease: a double-blind crossover pilot study. J Clin Neurosci 2018;47:72–78.ArticlePubMed
- 111. Ma J, Gao L, Mi T, Sun J, Chan P, Wu T. Repetitive transcranial magnetic stimulation does not improve the sequence effect in freezing of gait. Parkinsons Dis 2019;2019:2196195.ArticlePubMedPMC
- 112. Lee SY, Kim MS, Chang WH, Cho JW, Youn JY, Kim YH. Effects of repetitive transcranial magnetic stimulation on freezing of gait in patients with Parkinsonism. Restor Neurol Neurosci 2014;32:743–753.ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Libet’s legacy: A primer to the neuroscience of volition
Tomáš Dominik, Alfred Mele, Aaron Schurger, Uri Maoz
Neuroscience & Biobehavioral Reviews.2024; 157: 105503. CrossRef - Neural correlates of fine motor grasping skills: Longitudinal insights into motor cortex activation using fNIRS
Xiaoli Li, Minxia Jin, Nan Zhang, Wei Hongman, LianHui Fu, Qi Qi
Brain and Behavior.2024;[Epub] CrossRef - Affection of Motor Network Regions by Tau Pathology Across the Alzheimer's Disease Spectrum
Gérard N. Bischof, Elena Jaeger, Kathrin Giehl, Merle C. Hönig, Peter H. Weiss, Alexander Drzezga
eneuro.2024; 11(1): ENEURO.0242-23.2023. CrossRef - Parkinson’s Disease Risk Variant rs9638616 is Non-Specifically Associated with Altered Brain Structure and Function
Thomas Welton, Thomas Wei Jun Teo, Ling Ling Chan, Eng-King Tan, Louis Chew Seng Tan
Journal of Parkinson's Disease.2024; : 1. CrossRef - Sensorimotor network connectivity correlates with motor improvement after repetitive transcranial magnetic stimulation in patients with Parkinson's disease
Shumei Chi, Xinrui Wen, Yang Yu, Guanjun Wang, Jie Zhang, Chuang Xue, Xiaoying Zhang, Zheng Wang, Meiduo Gesang, Jiefang Chen, Sha Wu, Man Jin, Jian Liu, Benyan Luo
Parkinsonism & Related Disorders.2023; 106: 105218. CrossRef - Impaired topological properties of cortical morphological brain networks correlate with motor symptoms in Parkinson's disease
Su Yan, Jun Lu, Yuanhao Li, Tian Tian, Yiran Zhou, Hongquan Zhu, Yuanyuan Qin, Wenzhen Zhu
Journal of Neuroradiology.2023;[Epub] CrossRef - A new model for freedom of movement using connectomic analysis
Diego Alonzo Rodríguez-Méndez, Daniel San-Juan, Mark Hallett, Chris G. Antonopoulos, Erick López-Reynoso, Ricardo Lara-Ramírez
PeerJ.2022; 10: e13602. CrossRef - Cortical and subcortical morphological alterations in motor subtypes of Parkinson’s disease
Jianyu Li, Yuanchao Zhang, Zitong Huang, Yihan Jiang, Zhanbing Ren, Daihong Liu, Jiuquan Zhang, Roberta La Piana, Yifan Chen
npj Parkinson's Disease.2022;[Epub] CrossRef
Comments on this article
- Figure
- Related articles
-
- Impact of Deep Brain Stimulation on Non-Motor Symptoms in Parkinson’s Disease
- Cough as a presenting symptom in Wilson’s Disease
- Copper Deficiency Myeloneuropathy in a Patient With Wilson’s Disease
- A Survey of Perspectives on Telemedicine for Patients With Parkinson’s Disease
- Investigation of the Long-Term Effects of Amantadine Use in Parkinson’s Disease
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite