Articles
- Page Path
- HOME > J Mov Disord > Volume 17(1); 2024 > Article
-
Letter to the editor
Copper Deficiency Myeloneuropathy in a Patient With Wilson’s Disease -
Yu Wang1
 , Zijun Wei1
, Zijun Wei1 , Jianing Mei1
, Jianing Mei1 , Xueyi Han1
, Xueyi Han1 , Hongping Zhao1
, Hongping Zhao1 , Yulong Zhu2
, Yulong Zhu2 , Ping Jin2
, Ping Jin2 , Yunyun Zhang1
, Yunyun Zhang1

-
Journal of Movement Disorders 2024;17(1):123-126.
DOI: https://doi.org/10.14802/jmd.23188
Published online: November 22, 2023
1Department of Neurology, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
2Department of Neurology, The Affiliated Hospital of Institute of Neurology, Anhui University of Chinese Medicine, Hefei, Anhui, China
- Corresponding author: Yunyun Zhang, MD, PhD Department of Neurology, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, 110 Ganhe Road, Shanghai 200437, China / Tel: +86-18930569983 / E-mail: zhangyyshyy@shutcm.edu.cn
Copyright © 2024 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 805 Views
- 42 Download
- Dear Editor,
- Wilson’s disease (WD) is an autosomal recessive disorder of copper metabolism caused by mutations in the ATP7B gene. Pathogenic variants can lead to defects in or a loss of function of the P-type ATPase facilitating copper transport, resulting in a biliary copper excretion disorder [1]. Copper overload can lead to liver diseases, movement disorders, psychiatric symptoms, and Kayser-Fleischer rings (KFRs). WD can be treated, mainly through a low-copper diet and anti-copper drugs. However, long-term and excessive anti-copper treatment or a diet restricted in copper may result in copper deficiency. Here, we report the case of a patient in China who developed copper deficiency myeloneuropathy (CDM). The patient experienced refractory leukopenia and sensory-motor polyneuropathy, which improved after copper supplementation. Clinicians should be cautious about overcorrection when treating WD.
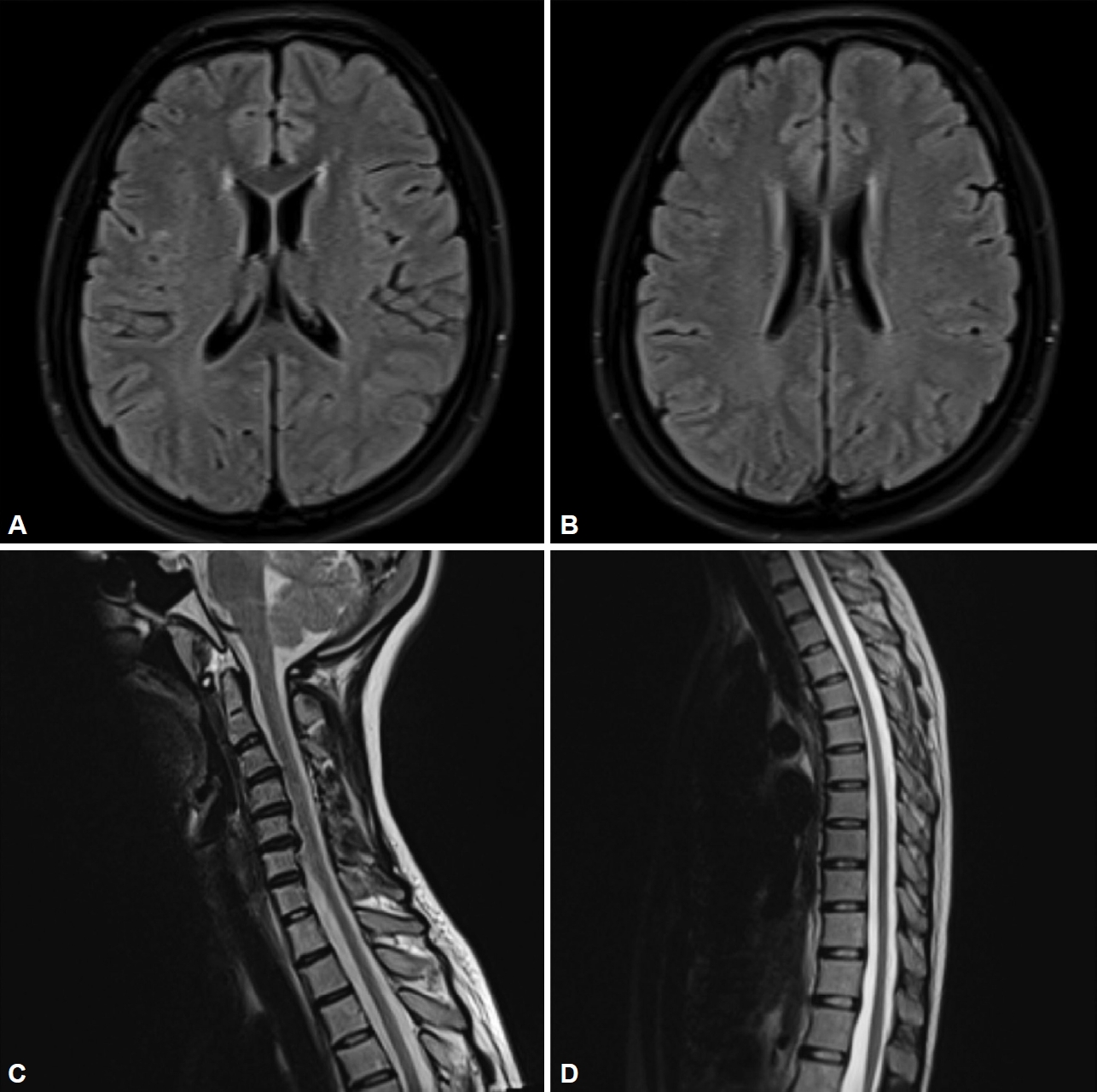
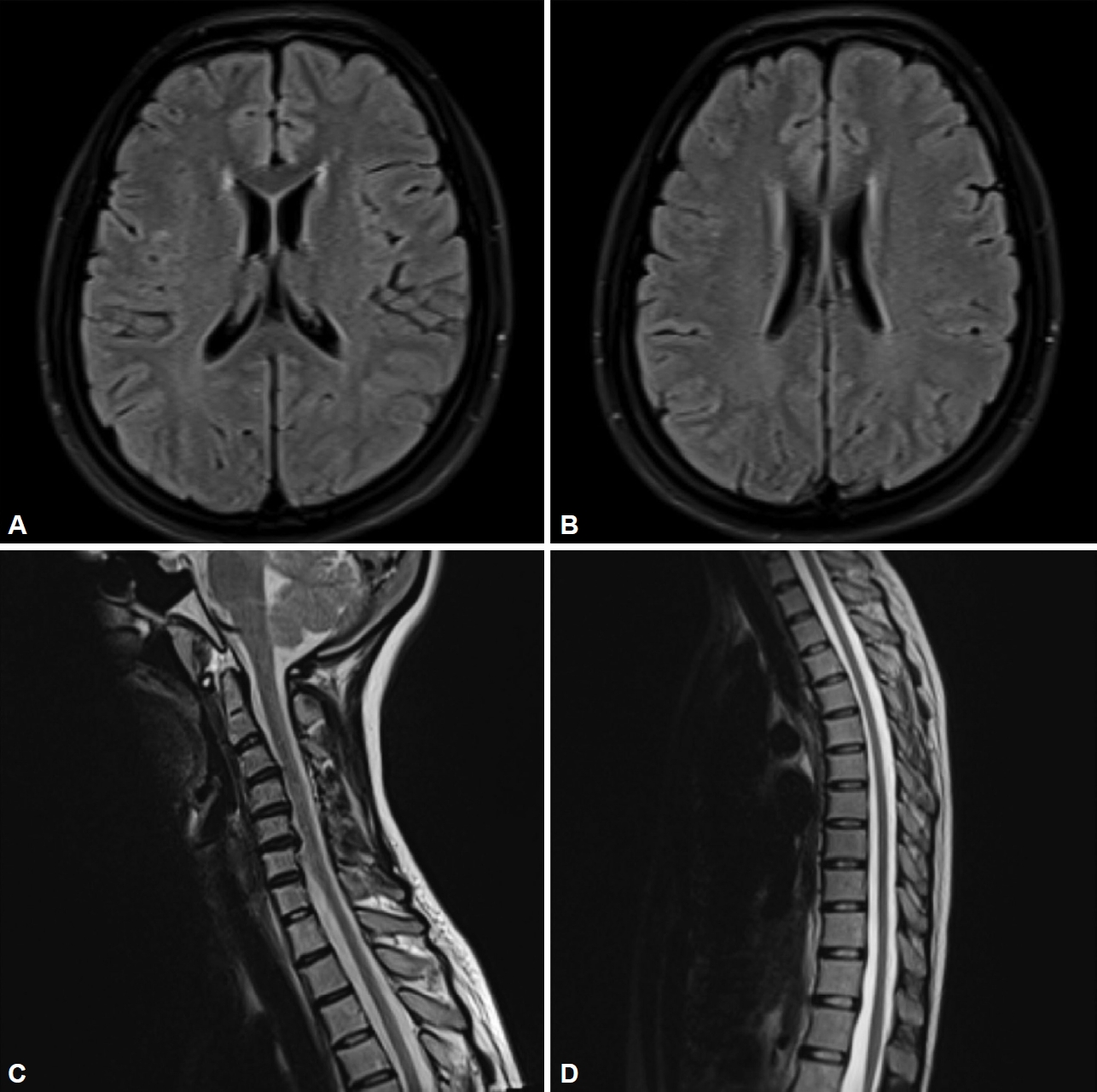
- A 30-year-old woman was diagnosed with WD 15 years prior because of dysarthria, dysphagia, KFRs, and hypoceruloplasminemia. She was started on a low-copper diet with intravenous infusion of sodium dimercaptopropanesulfonate (DMPS) at 1,000 mg/d, followed by zinc gluconate (ZnG) at 1,680 mg/d (equivalent to 240 mg of zinc). Additionally, 1,125 mg/d penicillamine (PCA) and 2,000 mg/d dimercaptosuccinic acid (DMSA) were taken alternately on a quarterly basis. Following treatment, the patient’s symptoms improved, allowing her to resume normal activities. Up until November 2019, the 27-year-old patient had exhibited leukopenia (2.78 × 109/L; normal range 3.5–9.5 × 109/L) accompanied by severe neutropenia (0.64 × 109/L; normal range 1.75–6.65 × 109/L). Moreover, her pinprick sensation and position sense gradually decreased in the distal limbs, and she displayed sensory gait ataxia (Supplementary Video 1 in the online-only Data Supplement) and pes arcuatus. As the test result for intrinsic factor antibody (IFA) was positive, the local hospital considered the existence of vitamin B12 binding or absorption disorders and diagnosed her with subacute combined degeneration (SCD); however, there was no improvement after vitamin B12 supplementation. By September 2022, the patient’s sensory impairment had worsened, leading to frequent falls during walking, and she experienced neuropathic pain in both feet. Electromyography (EMG) revealed axonal motorsensory polyneuropathy. No abnormalities were detected in the somatosensory-evoked potentials. Brain magnetic resonance imaging (MRI) showed mild demyelination near the lateral ventricles, whereas cervical and thoracic MRI showed no abnormalities (Figure 1). Laboratory and blood immunological tests did not reveal any antibodies related to peripheral neuropathy. Additionally, no abnormalities in folic acid, vitamin B12, homocysteine, thyroid hormones, infectious disease markers, or tumor-related markers were identified. Copper metabolism tests revealed significantly reduced serum levels of ceruloplasmin (CP) (28.4 mg/L; normal range 200–420 mg/L), copper oxidase (0.03 optical density [OD]; normal range > 0.2 OD), and total copper (1.01 μmol/L; normal range 10.5–24.4 μmol/L). Conversely, serum zinc levels were significantly increased (32.86 μmol/L; normal range 11.6–23 μmol/L). Furthermore, 24-hour urinary copper was measured at 179.86 μg/d (normal range < 100 μg/d), and 24-hour urinary zinc was significantly increased (9,150.21 μg/d). The patient’s parents were not consanguineous and reported no similar symptoms in the family. To eliminate the possibility of other hereditary peripheral neuropathies, such as Charcot-Marie-Tooth disease and ATP7A-related distal motor neuropathy, we performed whole-exome sequencing. This sequencing led to the identification of two heterozygous variants of ATP7B, namely, c.2662A>C:p.Thr888Pro and c.2590_2593dup:p.Thr865Serfs*3. The final diagnosis was a CDM secondary to WD. Anti-copper treatment was promptly discontinued, and a high-copper diet was initiated. At the February 2023 follow-up, the patient’s neuropathic pain had completely disappeared, and her gait disturbance had improved (Supplementary Video 2 in the online-only Data Supplement). Routine blood tests showed no leukopenia. The serum levels of CP, copper oxidase, and total copper were 30.3 mg/L, 0.032, and 1.02 μmol/L, respectively, with no significant changes over time. However, the serum zinc level dropped to normal levels at 12.6 μmol/L. The 24-hour urine copper level was 336.09 μg/d, and the 24-hour urine zinc level was 1,677.95 μg/d. The abnormal copper:zinc ratios in both the blood and urine tended to stabilize over time. EMG revealed that the motor and sensory nerve conduction velocities and amplitudes of both common peroneal nerves had returned to normal.
- Six male and nine female patients with CDM secondary to WD were previously reported in the literature (Supplementary Table 1 in the online-only Data Supplement). The disease onset was usually insidious, and the disease course was relatively long. It took approximately 17.9 ± 13.2 years (range 1–38 years) from WD diagnosis to the development of CDM. The risk factors for patients were long-term high-dose anti-copper therapy and a low-copper diet. For WD patients with neurological phenotypes in China, DMPS, DMSA, and ZnG were the initial treatment choice [2]. Moreover, traditional Chinese medicine was often used as an adjunctive therapy, and the diet plan was highly restrictive. The prevalence of WD in China is higher than that in European and American populations [2]. However, there is almost no research on CDM. Zhou et al. [2] reported that 33.5% of Chinese patients have poor treatment adherence, which could explain the scarcity of reports on CDM secondary to WD. IFA has been reported to cause various micronutrient absorption disorders [3]. Therefore, certain gastrointestinal diseases that affect nutrient absorption should also be considered risk factors for CDM.
- In the early stages of CDM secondary to WD, there may only be hematological symptoms, the most common of which are anemia and neutropenia, while a few patients may have thrombocytopenia. However, it can be clinically challenging to identify copper deficiency through routine blood tests [4]. Patients with WD may develop cirrhosis-related leukopenia and thrombocytopenia and drug-induced leukopenia. The typical neurological manifestation of CDM secondary to WD is sensory gait ataxia, which is caused by damage to the peripheral nerves or the posterior column. In patients in whom the lateral spinal cord is damaged, a spastic ataxic gait may occur. The symptoms of CDM secondary to WD are similar to those of SCD and must be distinguished from PCA-induced vitamin B6 deficiency, hepatic myelopathy, and late-onset Menkes disease. To determine the level of copper accumulation, it is essential to conduct trace element metabolism examinations. The reliability of the 24-hour urinary copper level measurement is affected by various factors. Laboratory testing for free copper has not been officially introduced in China, making it impossible to accurately determine the toxic copper levels. Additionally, the formula calculation method may yield false-negative results [5]. In our patient with CDM secondary to WD, copper metabolism test results indicated that serum total copper and 24-hour urinary copper levels were low, whereas serum zinc and 24-hour urinary copper levels were significantly increased. By dynamically comparing the copper:zinc ratios in the patient’s blood and urine, it is possible to determine the efficacy of anti-copper treatment [6]. EMG usually shows axonal motor-sensory polyneuropathy. Some patients may have abnormal posterior cord MRI signals [7], and a few may have periventricular demyelination changes on brain MRI.
- In conclusion, blindly relying on anti-copper treatment for WD can lead to iatrogenic copper deficiency in the long run. Copper supplementation treatment usually yields better outcomes in CDM secondary to WD, as hematological symptoms can be completely alleviated, and neurological damage is mostly reversible [5]. Regular monitoring of the copper:zinc ratio in patients with WD during treatment, along with individualized anti-copper and dietary plans, may help prevent or alleviate the damage caused by copper deficiency.
Supplementary Material
Video 1.
Video 2.
Supplementary Table 1.
-
Ethics Statement
The study received ethics approval from the Human Research Ethics Committee affiliated with the Affiliated Hospital of Institute of Neurology, Anhui University of Chinese Medicine, China (2022 No. 25). Written and informed consent was obtained for the written case report and video to be published.
-
Conflicts of Interest
The authors have no financial conflicts of interest.
-
Funding Statement
None
-
Author contributions
Conceptualization: Yu Wang. Data curation: Yu Wang, Jianing Mei, Xueyi Han, Hongping Zhao, Yulong Zhu, Ping Jin. Investigation: Yu Wang, Yulong Zhu, Ping Jin. Project administration: Zijun Wei. Supervision: Zijun Wei. Writing—original draft: Yu Wang. Writing—review & editing: Yunyun Zhang.
Notes
- We thank the patient for placing her trust in us.
Acknowledgments
- 1. Schilsky ML, Roberts EA, Bronstein JM, Dhawan A, Hamilton JP, Rivard AM, et al. A multidisciplinary approach to the diagnosis and management of Wilson disease: executive summary of the 2022 Practice Guidance on Wilson disease from the American Association for the Study of Liver Diseases. Hepatology 2023;77:1428–1455.ArticlePubMedPDF
- 2. Zhou ZH, Wu YF, Yan Y, Liu AQ, Yu QY, Peng ZX, et al. Persistence with medical treatment for Wilson disease in China based on a single center’s survey research. Brain Behav 2021;11:e02168. ArticlePubMedPMCPDF
- 3. Zilli A, Cavalcoli F, Ciafardini C, Massironi S. Deficiency of micronutrients in patients affected by chronic atrophic autoimmune gastritis: a single-institution observational study. Dig Liver Dis 2019;51:505–509.ArticlePubMed
- 4. Cortese A, Zangaglia R, Lozza A, Piccolo G, Pacchetti C. Copper deficiency in Wilson’s disease: peripheral neuropathy and myelodysplastic syndrome complicating zinc treatment. Mov Disord 2011;26:1361–1362.ArticlePubMedPDF
- 5. Litwin T, Antos A, Bembenek J, Przybyłkowski A, Kurkowska-Jastrzębska I, Skowrońska M, et al. Copper deficiency as Wilson’s disease overtreatment: a systematic review. Diagnostics 2023;13:2424.ArticlePubMedPMC
- 6. Duncan A, Talwar D, Morrison I. The predictive value of low plasma copper and high plasma zinc in detecting zinc-induced copper deficiency. Ann Clin Biochem 2016;53:575–579.ArticlePubMedPDF
- 7. da Silva-Júnior FP, Machado AA, Lucato LT, Cançado EL, Barbosa ER. Copper deficiency myeloneuropathy in a patient with Wilson disease. Neurology 2011;76:1673–1674.ArticlePubMed
REFERENCES
Figure & Data
References
Citations

Comments on this article
- Figure
- Related articles
-
- Caregiver Burden of Patients With Huntington’s Disease in South Korea
- A Survey of Perspectives on Telemedicine for Patients With Parkinson’s Disease
- Rapid-Onset Dystonia and Parkinsonism in a Patient With Gaucher Disease
- Hand Movement-Induced Eyeblink Bursts in a Patient With Parkinson’s Disease
- Umami and Other Taste Perceptions in Patients With Parkinson’s Disease
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite