Articles
- Page Path
- HOME > J Mov Disord > Volume 3(2); 2010 > Article
-
Case Report
Syndrome of Inappropriate Antidiuretic Hormone Secretion Associated with Pramipexole in a Patient with Parkinson’s Disease - Yoonjae Choi, Jeong Jin Park, Na Young Ryoo, So-Hyun Kim, Changseok Song, Im-Tae Han, Chang-Gi Hong, Choong Kun Ha, Seong Hye Choi
-
Journal of Movement Disorders 2010;3(2):54-56.
DOI: https://doi.org/10.14802/jmd.10015
Published online: October 30, 2010
Department of Neurology, Inha University School of Medicine, Incheon, Korea
- Corresponding author: Seong Hye Choi, MD, PhD, Department of Neurology, Inha University School of Medicine, 7-206 3-ga Sinheung-dong, Jung-gu Incheon 400-700, Korea, Tel 32-890-3659, Fax 32-890-3864, E-mail seonghye@inha.ac.kr
• Received: September 29, 2010 • Accepted: October 14, 2010
Copyright © 2010 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 28,094 Views
- 101 Download
- 4 Crossref
ABSTRACT
- The syndrome of inappropriate antidiuretic hormone secretion (SIADH) can be caused by a variety of drugs. Dopaminergic drugs might enhance the secretion of the antidiuretic hormone arginine vasopressin by reducing γ-amino butyric acid release through the dopaminergic receptor in supraoptic nucleus. A 75-year-old woman with Parkinson’s disease developed asthenia, delirium, aggravated parkinsonian symptoms, and hypotonic hyponatremia along with the diagnostic criteria for SIADH during dose escalation of pramipexole. After pramipexole withdrawal, these symptoms disappeared, and sodium levels returned to normal values. The serum sodium levels of patients receiving pramipexole should be monitored, especially during dose escalation.
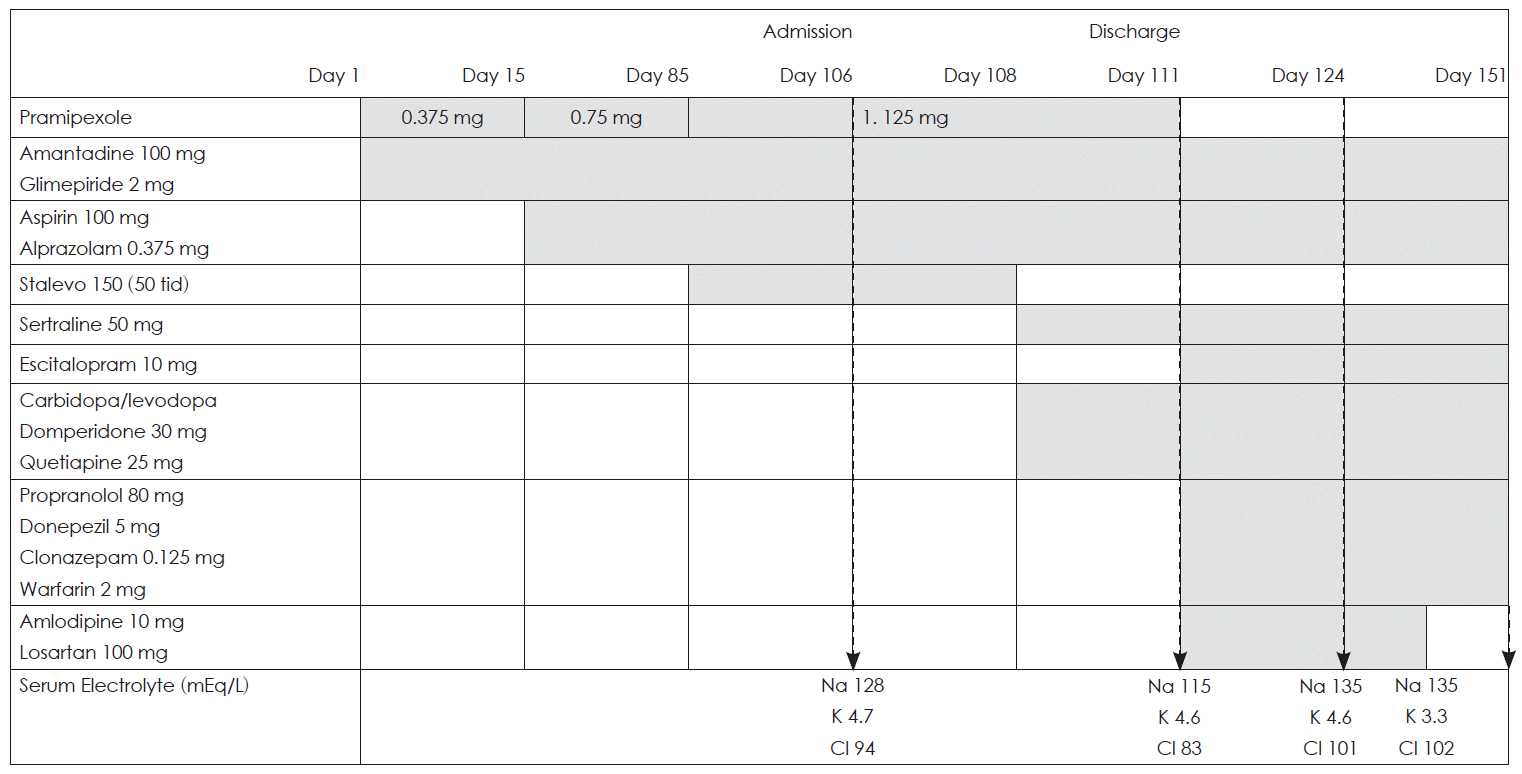
- A 75-year-old woman visited our neurology outpatient clinic because of feeling of leg weakness. She had chronic obstructive pulmonary disease and diabetes mellitus that was well controlled with 2 mg glimepiride. Upon neurological examination, she had a masked face, bradykinesia, a stooped posture, and limb rigidity that was more severe on the right side; she was diagnosed with Parkinson’s disease. The medication taken by this patient was summarized in Figure 1. Her treatment started with 100 mg amantadine and 0.375 mg pramipexole per day, and her clinical symptoms slightly improved. After two weeks, the dosage of pramipexole was increased to 0.75 mg per day, and 0.375 mg alprazolam and 100 mg aspirin per day were added because of anxiety and mild white matter changes on T2-weighted magnetic resonance images of the brain. After ten weeks, the dosage of pramipexole was increased to 1.125 mg per day, and levodopa/carbidopa/entacapone at 150/37.5/600 mg (Stalevo 50 tid) per day was added to treat postural instability and increased rigidity. After three additional weeks, she was admitted to our hospital because of asthenia, frequent falling, more aggravated rigidity, and dysarthria. Biochemical studies showed a serum sodium level of 128 mEq/L, a serum potassium level of 4.7 mEq/L, a serum chloride level of 94 mEq/L, a serum urea level of 23.1 mg/dL, and a serum creatinine level of 0.84 mg/dL. On the third day in the hospital, 50 mg sertraline was added. On the fourth day in the hospital, Stalevo was switched to carbidopa/levodopa at 37.5 mg/375 mg per day, and domperidone 30 mg per day was added. On the fifth day in the hospital, 25 mg quetiapine was added because of delirium. On the sixth day, sertraline was switched to 10 mg escitalopram, and biochemical studies showed a serum sodium level of 115 mEq/L, a serum potassium level of 4.6 mEq/L, a serum chloride level of 83 mEq/L, a serum osmolarity of 247 mOsm/kg, a urine osmolarity of 311 mOsm/kg, a urine sodium level of 56 mEq/L, a urine potassium level of 21.7 mEq/L, and a urine chloride level of 51 mEq/L. A thyroid function test was normal. She did not have any clinical evidence of adrenal insufficiency. She also did not have any hypervolemic features, such as subcutaneous edema, or any hypovolemic features, such as orthostatic hypotension, increased pulse rate, or dry mucous membranes. She did not have weight loss or any clinical symptoms that were related to malignancy. The serum levels of alpha-fetoprotein, cancer antigen-125, carbohydrate antigen 19-9, and beta-human chorionic gonadotropin were in normal range. She was diagnosed with SIADH possibly induced by a drug. The clinical symptoms and the serum sodium levels improved after stopping pramipexole. On the 19th day in the hospital, which was also the day of discharge, her medication consisted of 100 mg amantadine, carbidopa/levodopa at 225 mg/1,125 mg, 30 mg domperidone, 80 mg propranolol, 25 mg quetiapine, 5 mg donepezil, 10 mg escitalopram, 2 mg glimepiride, 0.125 mg clonazepam, 0.375 mg alprazolam, 100 mg aspirin, 10 mg amlodipine, 100 mg losartan, and 2 mg warfarin per day because of deep vein thrombosis, and biochemical studies showed a serum sodium level of 135 mEq/L, a serum potassium level of 4.6 mEq/L, and a serum chloride level of 101 mEq/L. Two weeks after discharge, amlodipine and losartan were stopped because of low blood pressure. On the 27th days after discharge, biochemical studies showed a serum sodium level of 135 mEq/L, a serum potassium level of 3.3 mEq/L, and a serum chloride level of 102 mEq/L.
Case
- Our patient developed SIADH 21 days after increasing the dosage of pramipexole, and SIADH disappeared after pramipexole withdrawal. The temporal relationship between the development/disappearance of SIADH and the pramipexole dosage suggests a possible cause-effect relationship between pramipexole and SIADH.
- The development of SIADH associated with amantadine has been rarely reported.4,5 Although our patient continued to take amantadine, SIADH disappeared after pramipexole withdrawal. Therefore, SIADH was associated with parmipexole in our patient. SSRIs can also induce SIADH.3 Our patient took sertraline after the development of hyponatremia, and sertraline was switched to escitalopram without a washout period. Pramipexole withdrawal induced the disappearance of SIADH in spite of her taking an SSRI. Therefore, SIADH was not associated with SSRIs in our patient.
- Arginine vasopressin (AVP) is induced by noradrenalin and serotonin through adrenergic α1 receptor and 5-HT2A/2C receptors in supraoptic nucleus.6,7 AVP-secreting supraoptic neurons are under the control of prominent γ-amino butyric acid (GABA) inhibition, and D4 receptors are located on GABA terminals in the rat supraoptic nucleus.6 Dopaminergic activation via D4 receptors reduces GABA release in the supraoptic nucleus, and then AVP release is facilitated.6 Pramipexole has a high affinity for the D2, D3 and D4 receptors.7,8 Therefore, a possible explanation for SIADH in this patient is the pramipexole-enhanced AVP secretion resulting from reducing GABA release through D4 receptors in the supraoptic neurons. Pramipexole has a higher selectivity for D4 receptor than other dopamine agonists.9 Thus, it may be more likely to increase AVP secretion compared with other dopamine agonists.
- This case had some limitations. Although she did not have any clinical evidence of adrenal insufficiency or malignancy, we did not perform adrenal function test and thorough diagnostic work-up to exclude adrenal insufficiency and malignancy. We did not think that drug interactions between pramipexole and other drugs contributed to inducing SIADH in this case because 90% of pramipexole is excreted unchanged in the urine.9 However, the synergistic effect of amantadine and pramipexole on inducing SIADH could not be excluded in this case.
- In this case, the SIADH induced rapid aggravation of parkinsonian symptoms such as falling, rigidity, and dysarthria, but those symptoms were fully recovered as correcting hyponatremia. Clinicians should consider medical derangement when a rapid aggravation of parkinsonian symptoms is developed in a patient with Parkinson’s disease. Recently, two cases with SIADH induced by pramipexole were reported.7,10 In one case, SIADH developed during first two weeks of pramipexole therapy,10 and in the other case, SIADH developed during dose escalation of pramipexole,7 as with our patient.
- Pramipexole might facilitate AVP secretion in some patients, which is a prerequisite for developing SIADH. The serum sodium levels of patients receiving pramipexole should be monitored, especially in the first few weeks after starting therapy and during dose escalation.
Discussion
- This study was supported by a grant of the Inha Univeristy School of Medicine, Incheon, Republic of Korea.
Acknowledgments
Acknowledgments
Figure 1Overview of medication taken by this patient. The dosages are the amount taken daily. The gray-colored columns mean the durations of taking drugs.

- 1. Upadhyay A, Jaber BL, Madias NE. Epidemiology of hyponatremia. Semin Nephrol 2009;29:227–238.ArticlePubMed
- 2. Fenske W, Allolio B. The syndrome of inappropriate secretion of antidiuretic hormone: diagnostic and therapeutic advances. Horm Metab Res 2010;42:691–702.ArticlePubMed
- 3. Ellison DH, Berl T. Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med 2007;356:2064–2072.ArticlePubMed
- 4. van Laar T, Lammers GJ, Roos RA, Gerritsen JJ, Meinders AE. Antiparkinsonian drugs causing inappropriate antidiuretic hormone secretion. Mov Disord 1998;13:176–178.ArticlePubMed
- 5. Alonso Navarro H, Sánz-Aiz A, Izquierdo L, Jiménez Jiménez FJ. Syndrome of inappropriate antidiuretic hormone secretion possibly associated with amantadine therapy in Parkinson disease. Clin Neuropharmacol 2009;32:167–168.ArticlePubMed
- 6. Azdad K, Piet R, Poulain DA, Oliet SH. Dopamine D4 receptor-mediated presynaptic inhibition of GABAergic transmission in the rat supraoptic nucleus. J Neurophysiol 2003;90:559–565.ArticlePubMed
- 7. Arai M, Iwabuchi M. Pramipexole as a possible cause of the syndrome of inappropriate antidiuresis. BMJ Case Reports 2009;10.1136/bcr.01.2009.1484. ArticlePubMedPMC
- 8. Mierau J, Schneider FJ, Ensinger HA, Chio CL, Lajiness ME, Huff RM. Pramipexole binding and activation of cloned and expressed dopamine D2, D3 and D4 receptors. Eur J Pharmacol 1995;290:29–36.ArticlePubMed
- 9. Kvernmo T, Härtter S, Burger E. A review of the receptor-binding and pharmacokinetic properties of dopamine agonists. Clin Ther 2006;28:1065–1078.ArticlePubMed
- 10. Tomita M, Otsuka Y, Iida R, Kobayashi S, Kuriyama S, Hosoya T. Syndrome of inappropriate ADH secretion (SIADH) induced by pramipexole in a patient with Parkinson’s disease. Nippon Jinzo Gakkai Shi 2005;47:531–535.PubMed
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Syndrome of Inappropriate Antidiuretic Hormone Secretion as a Presentation of Untreated Parkinson’s Disease
Karim Ali, Yaseen Najjar, Swati Mehta , Geovani Faddoul
Cureus.2023;[Epub] CrossRef - Is Restless Legs Syndrome De Facto Thyroid Disease?
Szymon Suwała, Jakub Rzeszuto, Rafał Glonek, Magdalena Krintus, Roman Junik
Biomedicines.2022; 10(10): 2502. CrossRef - Levodopa-carbidopa intestinal gel is an option in Parkinson’s disease with hyponatremia induced by dopamine agonists
Marina Picillo, Vincenzo Canoro, Giulio Cicarelli, Roberto Erro, Paolo Barone
Neurological Sciences.2020; 41(11): 3361. CrossRef - Liquid Levodopa/Carbidopa: Old Solution, Forgotten Complication
Nirosen Vijiaratnam, Shuli Cheng, Kelly Lucinda Bertram, David Richard Williams
Journal of Movement Disorders.2017; 10(3): 164. CrossRef
Comments on this article
- Figure
- Related articles
-
- Potential Link Between Cognition and Motor Reserve in Patients With Parkinson’s Disease
- Hand Movement-Induced Eyeblink Bursts in a Patient With Parkinson’s Disease
- Umami and Other Taste Perceptions in Patients With Parkinson’s Disease
- Constipation is Associated With Mild Cognitive Impairment in Patients With de novo Parkinson’s Disease
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite

